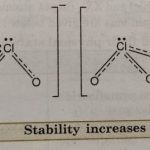
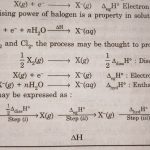Oxoacids of Halogens Among the halogens, fluorine has very little tendency to form oxoacids due to its high electronegativity and small size. However, it forms one oxoacid HOF known as fluoric (I) acid or hypofluorous acid. The rest of the halogens form four series of oxoacids, HOX, HXO2, HXO3, and HXO4. Most of these cannot be isolated in pure state. They are stable only in … [Read more...] about Oxoacids of Halogens and Interhalogen Compounds
Class 12
Hydrogen Chloride
Hydrogen Chloride Preparation Laboratory Preparation of Hydrogen Chloride Hydrogen chloride is prepared by heating sodium chloride with concentrated sulphuric acid. NaCl + H2SO4 -----> NAHSO4 + HCl NaCl + NaHSO4 ------> Na2SO4 + HCl HCl gas is dried by passing through concentrated sulphuric acid. On Industrial scale hydrochloric acid is manufactured by heating a … [Read more...] about Hydrogen Chloride
Chlorine
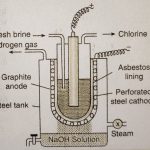
Chlorine Occurrence Chlorine is very reactive and does not occur in nature in free state. It occurs mostly as chlorides of sodium and other alkali and alkaline earth metals. The most abundant compound of chlorine is sodium chloride (rock salt) and occurs in extensive evaporite deposits, saline lakes and brines and in the ocean. Other sources of chlorine are sylvine (KCl), … [Read more...] about Chlorine
Chemical Properties of Group 17 Elements
Chemical Reactivity The halogens react readily with metals and non-metals to form halides. Fluorine is the most reactive of all the halogens. The reactivity of the halogens decreases down the group. The high reactivity of halogens is due to the following reasons : (i) Low dissociation enthalpies All the halogens have very low dissociation enthalpies. As a result, … [Read more...] about Chemical Properties of Group 17 Elements
Physical Properties of Group 17 elements
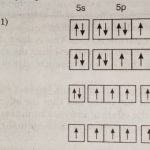
Group 17 Elements Group 17 of the periodic table contains five elements : fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). These are named as halogens. The name halogens is derived from two Greek words halo meaning sea salt and gens meaning born i.e., sea salt produce because the first three members occur as salts (chlorides, bromides and iodides) in … [Read more...] about Physical Properties of Group 17 elements