Isomerism
Two or more compounds having the same molecular formula but different chemical and physical properties are called isomers and the phenomenon is known as isomerism.
It is of 2 types:
1) Structural isomerism
2) Stereoisomerism
1) Structural Isomerism : Compounds having the same molecular formula but different structures i.e. different arrangement of atoms within the molecules are called structural isomers and the phenomenon is called structural isomerism.
It is of 6 types:
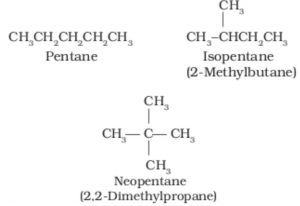
1)Chain or nuclear isomerism: Compounds having the same molecular formula but different arrangement of carbon chain within the molecule are called chain or nuclear isomers and the phenomenon is called chain or nuclear isomerism.
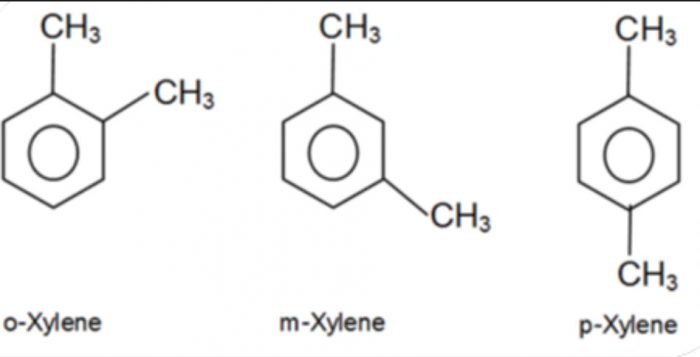
2) Position isomerism: Compounds which have the same structure of the carbon chain but differ only in the position of the multiple bond or functional group are called position isomers and the phenomenon is called position isomerism.
3) Functional isomerism
Compounds having same molecular formula but different functional group are called functional isomers and the phenomenon is called functional isomerism.
1) Alcohols and ethers
2) Carboxylic acid and esters
3) Aldehydes , ketones , unsaturated alcohols and unsaturated ethers
CH3-CO-CH3 Propanone
CH3CH2-CHO Propanal
CH2=CH-CH2OH Prop-2-en-1-ol
CH2=CH-O-CH3 Methoxyethene
4) Aromatic alcohols, phenols, ethers
5) Dienes , allenes and alkynes
CH2=CH-CH=CH2 Buta-1,3-diene
CH2=C=CH-CH3 Buta-1,2-diene
CH3CH2C≡CH But-1-yne
CH3CH2C≡CCH3 But-2-yne
6) Nitroalkanes ,alkyl nitrites and amino acids (CnH2n+1NO2)
CH3CH2NO2 Nitroethane
CH3-CH2-O-N=O Ethyl nitrite
H2N-CH2-COOH 2-Aminoethanoic acid
7) 1° , 2° and 3° amines
CH3CH2CH2NH2 Propan-1-amine
CH3CH2-NH-CH3 N-Methylethanamine
CH3-N-(CH3)2
N,N-Dimethylmethanamine
8) Cyanides and Isocyanides
CH3C≡N Ethanenitrile
CH3-NC Methyl isocyanide
4) Metamerism
Compounds having the same molecular formula but different number of carbon atoms on either side of the functional group are called metamers and the phenomenon is called metamerism. Metamerism occurs among the members of the same homologous family.
1) CH3CH2-O-CH2CH3 (Ethoxyethane) is a metamer of
CH3-O-CH2CH2CH3 (1-Methoxypropane) or CH3O-CH(CH3)2 (2-Methoxypropane)
2) CH3CH2-S-CH2CH3 (Diethyl thioether) is a metamer of
CH3-S-CH2CH2CH3 (Methyl propyl thioether) or CH3S-CH(CH3)2 (Isopropyl methyl thioether)
3) CH3CH2-NH-CH2CH3 (Diethylamine)
is a metamer of CH3-NH-CH2CH2CH3 (Methyl propylamine) or CH3-NH-CH(CH3)2 (Isopropyl methylamine)
4) CH3COOCH2CH3 (Ethyl acetate) and CH3CH2COOCH3 (Methyl propionoate) are metamers.
5) Tautomerism
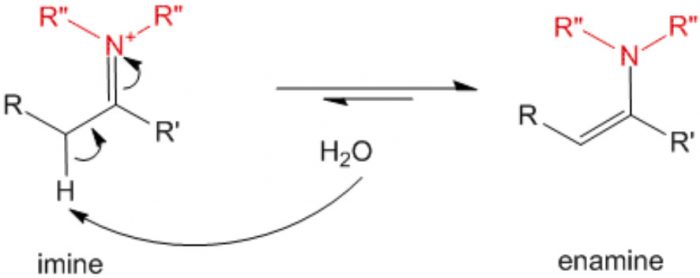
In this isomerism the isomers exist in dynamic equilibrium with each other.It arises due to migration of a hydrogen atom from one polyvalent atom to the other within the same molecule with necessary rearrangement of linkages.The isomers thus obtained are called tautomers and the phenomenon is called tautomerism.
Tautomerism is also called desmotropism.
The two types of tautomerism are:
Dyad system : This type of tautomerism involves 1,2- migration of a proton from one polyvalent atom to the other within the same molecule with necessary rearrangement of linkages.
HCN ⇔ HNC
Hydrogen migrates from carbon to nitrogen.The alkyl derivative of these tautomers are called cyanide and isocyanide.
RCN Alkyl cyanide
RNC Alkyl isocyanide
Triad system
Hydrogen migrates from one polyvalent atom to the third polyvalent atom within the same molecule.
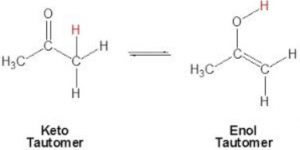
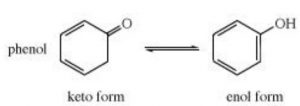
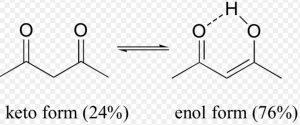
Keto-enol tautomerism is just one type of triad system.One form contains keto group while the other contain the enolic group.
In all the monocarbonyl compounds, the greater stability of the keto form w.r.t the enol form is due to the greater strength of the carbon -oxygen π bond as compared to carbon-carbon π bond.
Factors affecting the relative amount of keto and enol forms in keto-enol tautomerism
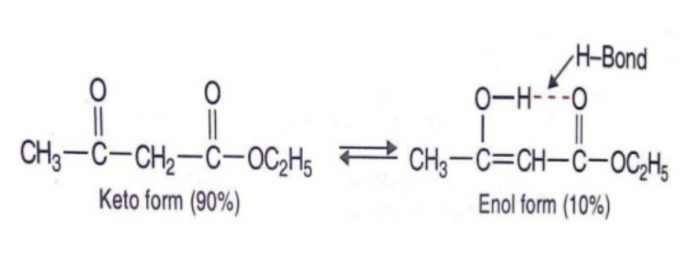
- Stability of the enol form: In simple aldehydes and ketones the amount of enolic form is negligibly small.If the enolic form is stabilized by intramolecular hydrogen – bonding or resonance, such as in 1,3-dicarbonyl compounds, the amount of enolic form is much greater than in acetaldehyde or acetone.
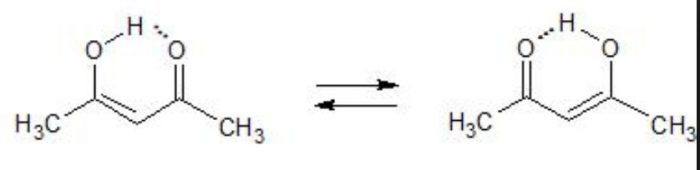
Acetylacetone also exhibit also exhibit keto-enol tautomerism but the amount of enolic form here is much higher than even in acetoacetic ester .This is due to the reason that keto group is a much better electron withdrawing group than the ester group.
Higher the stability of the enol form , greater is the enol content.
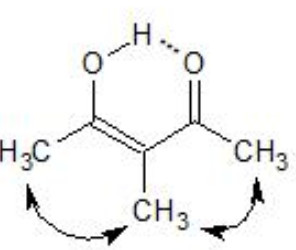
2) Steric hindrance
Acetylacetone
α-Methylacetylacetone
Both the enols are stabilized by H-bonding , the enol form of α-methylacetylacetone is destabilized to some extent by the steric repulsion due to the presence of the α-methyl group.
As a result α-methylacetylacetone has lower enol content as compared to that of acetylacetone.
3)Effect of polarity of solvent
Polar protic solvents such as water, methanol , acetic acid which form H-bonds with the carbonyl group of the keto form decrease the enol content. Aprotic solvents such as hexane, benzene increase the enol content.
Essential condition
- The compound must have a electronegative atom (N,O,S) bonded by a double or a triple bond.
2 The compound must have atleast one acidic α-hydrogen present on a saturated carbon. Acetophenone , butan-2-one and propionaldehyde all contain acidic α-hydrogen atoms and hence show keto-enol tautomerism.
C6H5COCH3 Aectophenone
CH3COCH2CH3 Butan-2-one
CH3CH2CHO Propionaldehyde
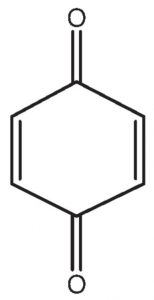
p-benzoquinone contain α-hydrogens but they are not acidic because they are present on a double bond.Therefore it does not show keto-enol tautomerism.
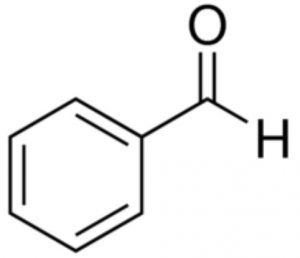
Benzaldehyde
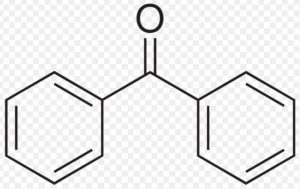
Benzophenone
Benzophenone and benzaldehyde do not show keto-enol tautomerism because they do not contain α-hydrogen atoms.
Other types of tautomerism involving 1,3-hydrogen shifts are:
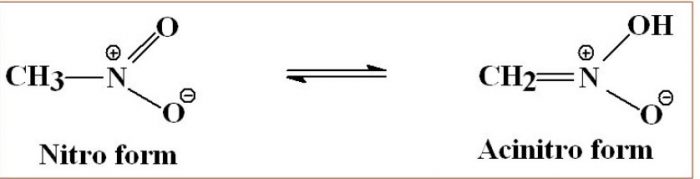
- Nitro- acinitro tautomerism : 1° and 2° nitroalkanes which contain acidic α- hydrogen show tautomerism.
2 Nitroso- oximino system :
3 Imine-enamine system
6 Ring-Chain isomerism
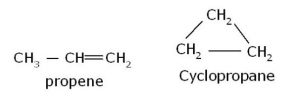
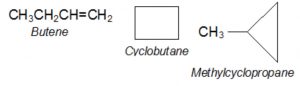
Compounds having the same molecular formula but possessing open chain and cyclic structures are called ring chain isomers and the phenomenon is called ring-chain isomerism.
- Alkenes and cycloalkanes
2 Alkynes and cycloalkenes
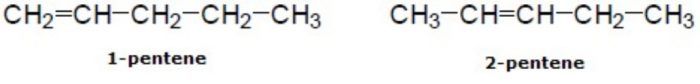
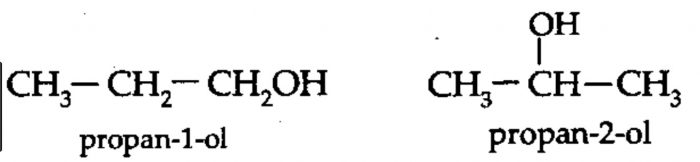


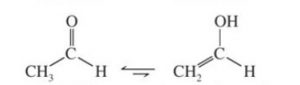
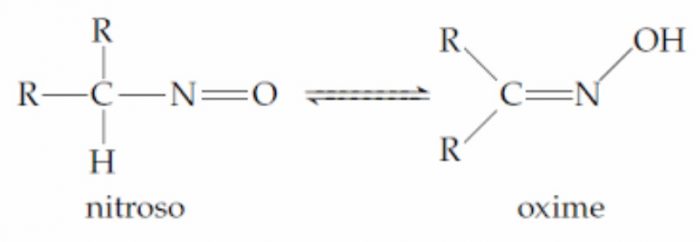

Very happy to refresh what i studied long long ago. Nicely explained.
Very nice
When I studied i am feeling refresh on this topic
it is a very good summary but mam you should include some more examples.
Thank you mam its really very very useful for me
excellet notes. Can we get these in pdf or printable format??