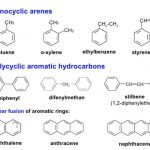
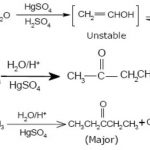
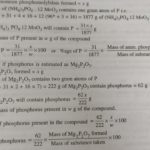Hydrocarbons and their alkyl, alkenyl and alkynyl derivatives which contain one or more benzene rings either fused or isolated in their molecules are called aromatic hydrocarbons. Benzene ring is highly unsaturated and in most of the reactions of aromatic compounds, the unsaturation of benzene ring is retained. Aromatic hydrocarbons containing a benzene ring are called … [Read more...] about Arenes
Class 11
Alkynes
Alkynes or Acetylene Acyclic unsaturated hydrocarbons containing a carbon-carbon triple bond are called alkynes or acetylenes. Their general formula is CnH2n-2 where n=2,3,4....etc. Structure of Triple bond Ethyne is the first member of alkynes series. Each carbon atom of ethyne is sp-hybridized and hence has two sp-hybridized orbitals. One sp-hybridized orbital … [Read more...] about Alkynes
Alkenes
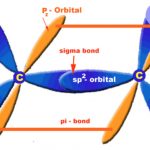
Acyclic unsaturated hydrocarbons containing a carbon-carbon double bond are called alkenes. They are also called olefins since the lower members of this class produce oily products on reaction with halogens such as chlorine and bromine. Structures of Double Bond The carbon-carbon double bond in alkenes consists of one strong carbon-carbon σ-bond with a bond dissociation … [Read more...] about Alkenes
Quantitative Analysis
Quantitative analysis means to determine the percentage of each element. Estimation of Carbon and Hydrogen Principle. A known mass of the organic compound is heated strongly with excess of dry copper oxide in a current of dry air or oxygen (free from carbon dioxide). Under these conditions, carbon present in the organic compound is oxidized to carbon dioxide and hydrogen is … [Read more...] about Quantitative Analysis
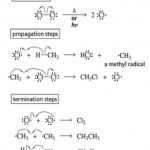
Alkanes
Hydrocarbons are divided into two types: (1) Acyclic or open chain hydrocarbons (2) Cyclic or closed chain hydrocarbons Acyclic or open chain hydrocarbons These compounds contain open chains of carbon atom in their molecules. They are also called aliphatic hydrocarbons. These are further classified into following three categories : (1) Alkanes : An alkane has only … [Read more...] about Alkanes