Contents
- 1 Alkynes or Acetylene
- 2 Structure of Triple bond
- 3 Isomerism in Alkynes
- 4 Classification of Alkynes
- 5 Methods of preparation of Alkynes
- 6 Physical properties of Alkynes
- 7 Reactivity of alkynes versus Alkenes
- 8 Chemical Reaction Of alkynes
- 9 Electrophilic Addition Reactions
- 10 Reduction of Alkynes
- 11 Oxidation Reaction of Alkynes
- 12 Uses of Alkynes
Alkynes or Acetylene
Structure of Triple bond
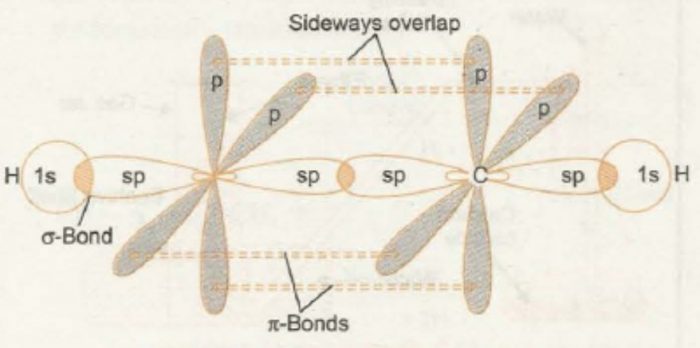
Ethyne is the first member of alkynes series.
Each carbon atom of ethyne is sp-hybridized and hence has two sp-hybridized orbitals. One sp-hybridized orbital of each carbon undergoes head on overlap with sp-hybridized orbital of another carbon to form a sp-sp, C-C, σ – bond.
The second sp-hybridized orbital of each carbon overlaps along the internuclear axis with 1s-orbital of each of the two hydrogen atoms forming two sp-s, C-H, σ-bonds. Each carbon is now left with two unhybridized p-orbitals (2px and 2py) which are perpendicular to each other.
The two 2px-orbitals, one on each carbon, are parallel to each other and hence overlap sideways to form a π bond. Similar overlap between 2py orbitals, one on each carbon, results in the formation of a second π bond.
Since a p-orbital has two lobes, the electron cloud of a π-bond has two halves. If the two halves of one π bond are considered to lie in the plane of the paper, then one of the two halves of the second π-bond would lie above the plane of the paper and the other below the plane of the paper.
The four halves of the electron clouds of two π-bonds do not stay as such but merge together to form a single electron cloud which has cylindrical symmetry about the internuclear axis. It is because of this cylindrical symmetry of the electron cloud between two carbon atoms that ethyne is a linear molecule with H-C-C bond angle of 180°.
Carbon carbon triple bond consists of one strong σ – bond and two weak π-bonds. Due to smaller size of sp-orbitals and sideways overlap of p-orbitals, the carbon-carbon bond length in ethyne is shorter than those of C = C and C -C .
Alkynes are less reactive than alkenes towards addition reactions. Alkynes unlike alkenes do not exhibit geometrical isomerism due to their linear structure.
Isomerism in Alkynes
Position isomerism
CH3-CH2-C≡CH But-1-yne
Chain isomerism
Alkynes having or five or more carbon atoms show isomerism due to different structures of the carbon chain.
CH3-CH2-CH2-C≡CH Pent-1-yne
3-Methylbut-1-yne
Functional isomerism
Alkynes are functional isomers of dienes i.e. compounds containing two double bonds.
CH3-CH2-C≡CH But-1-yne
CH2=CH-CH=CH2 But-1,3-dienes
CH2=C=CH-CH3 But-1,2-diene
Ring chain isomerism
Alkynes show ring chain isomerism with cycloalkenes.
CH3-C≡CH Propyne
Classification of Alkynes
Alkynes are further classified as terminal or non-terminal alkynes according as the triple bond is present at the carbon chain or within the carbon chain.
Terminal alkynes
CH3C≡CH Propyne
CH2-CH2-C≡CH But-1-yne
Non-Terminal alkynes
CH3-C≡C-CH2-CH3 Pent-2-yne
Methods of preparation of Alkynes
1.By the action of water on calcium carbide
Ethyne (acetylene) is prepared in the laboratory as well as on a commercial scale by the action of water on calcium carbide.
CaC2 + 2 H2O → HC≡CH + Ca(OH)2
Calcium carbide needed for the purpose is manufactured by heating limestone (calcium carbonate) with coke in an electric furnace at 2275 K.
CaCO3 → CaO + CO2
Lumps of calcium carbide are placed on a layer of sand in a conical flask fitted with a dropping funnel and a delivery tube. The air present in the flask is replaced by oil gas since acetylene forms an explosive mixture with air. Water is now dropped from dropping funnel and the acetylene gas thus formed is collected over water.
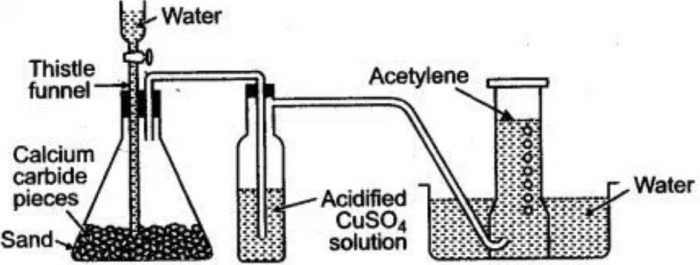
Acetylene gas contains impurities of hydrogen sulphide and phosphine due to the contaminations of calcium sulphide and calcium phosphide in calcium carbide. Hydrogen sulphide is removed by bubbling the gas through an acidified solution of copper sulphate while phosphine is removed by passing the gas through an acidified solution of copper sulphate while phosphine is removed by passing the gas through a suspension of bleaching powder. Pure acetylene is finally collected over water.
2. By dehydrohalogenation of dihaloalkanes
Alkynes are prepared by dehydrohalogenation of vicinal dihaloalkanes by heating them with an alcoholic solution of potassium hydroxide.
BrCH2-CH2Br +2 KOH(alc) → CH≡CH +2 KBr + 2 H2O
BrCH2-CH2Br +KOH(alc) → CH2=CHBr + KBr + H2O
In ethylene dibromide, Br is present on a saturated carbon atom. Therefore, like alkyl halides, it is a reactive molecule. On heating with alcoholic KOH, it readily eliminates a molecule of HBr to form vinyl bromide in good yield. Due to the presence of Br on a doubly bonded carbon atom, vinyl bromide is a highly unreactive molecule and hence on heating with alcoholic KOH, it does not easily loss a molecule of HBr to form acetylene.
With alcoholic KOH, the yield of acetylene is low. Therefore to obtain acetylene in fairly good yield from vinyl bromide, a much stronger base than alcoholic KOH such as NaNH2 in liquid NH3 is usually used. Dehydrohalogenation of ethylene dibromide to acetylene is preferably carried out in the following two stages.
The reaction is usually carried out in one step using NaNH2 in liquid NH3.
BrCH2-CH2Br + 2 NaNH2 → CH≡CH + 2NaBr +2 NH3
CH3-CHBr2 → CH2=CHBr in presence of KOH(alc)
CH2=CHBr → HC≡CH in presence of NaNH2 / liq NH3
3. By dehalogenation of terahalides
1,1,2,2-tetrabromoethane
4. By dehalogenation of haloforms
CHCl3 +6 Ag + Cl3CH → HC≡CH + 6 AgCl
5. Kolbe’s electrolytic reaction
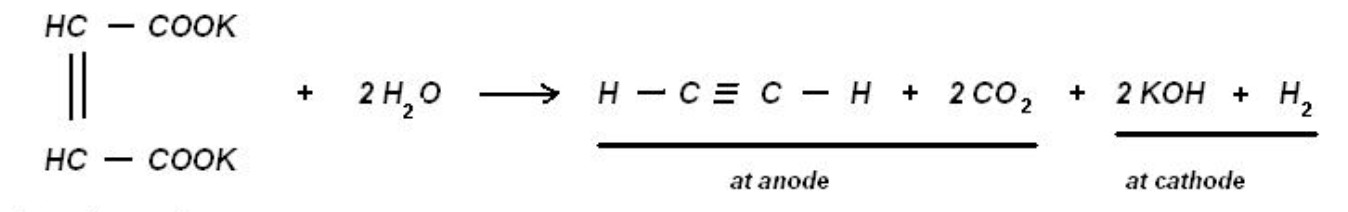
6. Synthesis from carbon and hydrogen
7. Synthesis of higher alkynes from acetylene
HC≡CH + NaNH2 → HC≡C¯Na+ + NH3
HC≡C¯Na+ +CH3Br → HC≡C-CH3 + NaBr
HC≡C¯Na+ + CH3CH2-I → HC≡C-CH2CH3 + NaI
Physical properties of Alkynes
(i) Physical state: The first three members of this family (ethyne, propyne and butyne) are colourless gases, the next eight are liquids while the higher ones are solids.
(ii) Smell: All the Alkynes are odourless. However, acetylene has garlic smell due to the presence of phosphine as impurity.
(iii) Melting and boiling points The melting points and boiling points of alkynes are slightly higher than those of the corresponding alkenes. This is probably due to the reason that because of the presence of a triple bond, alkynes have linear structures and hence their molecules can be more closely packed in the crystal lattice as compared to those of corresponding alkenes and alkanes.
(iv) Solubility Alkynes like alkanes being non – polar are insoluble in water but readily dissolve in organic solvents such as petroleum ether, benzene, carbon, tetrachloride, etc.
(v) Density Densities of alkynes like those of alkenes and alkanes increase as the molecular size increases. However, they are all lighter than water since their densities lie in the range 0.69 – 0.77 g/cm3.
Reactivity of alkynes versus Alkenes
Chemical Reaction Of alkynes
Acidic character of Alkynes
The hydrogen atoms attached to the the triple bond of alkynes are acidic in nature.
(1) Formation of alkali metal acetylides : Ethyne and other terminal alkynes or 1-alkynes react with strong bases such as sodium metal at 475 K or sodamide in liquid ammonia at 196 K to form sodium acetylides with evolution of H2 gas.
2HC≡CH +2 Na → 2CH≡C¯Na+ + H2
R-C≡CH + NaNH2 → R-C≡C¯Na+ + NH3
During these reactions, the acetylenic hydrogen is removed as a proton to form stable carbanions.
Sodium acetylide is decomposed by water regenerating acetylene. This shows that water is stronger acid than acetylene and thus displaces acetylene from sodium acetylene.
HC≡C¯Na+ + H2O → HC≡CH + NaOH
(2) Formation of heavy metal acetylene : Acetylenic hydrogens of alkynes can also be replaced by heavy metal ions such as Ag+ and Cu+ ions. When treated with ammoniacal silver nitrate solution (Tollen’s reagent), alkynes form white precipitate of silver acetylides.
CH≡CH + 2[Ag(NH3)2]+OH¯ → AgC≡CAg + 2 H2O + 4 NH3
With ammoniacal cuprous chloride solution, terminal alkynes form red ppt. of copper acetylides.
HC≡CH + 2[Cu(NH3)2]+OH¯ → CuC≡C-Cu + 2 H2O + 4 NH3R-C≡CH + [Cu(NH3)2]+OH¯ → R-C≡C-Cu +H2O + 2 NH3
Silver and copper acetylides are not decomposed by water. They can however, be decomposed with dilute mineral acids to regenerate the original alkynes.
AgC≡CAg +2 HNO3 → HC≡CH + 2 AgNO3
(3) Formation of alkynyl Grignard reagent : Acetylene and other terminal alkynes react with Grignard reagents to form the corresponding alkynyl Grignard reagents.
HC≡CH + RMgX → HC≡CMgX + RH
(i) For separation and purification of terminal alkynes from non terminal alkynes, alkanes and alkenes.(ii) To distinguish terminal alkynes from non terminal alkynes or alkenes.
Electrophilic Addition Reactions
The electrophilic addition reactions of alkynes take place in two steps in two stages as shown below :
HC≡CH + CCl2 → ClHC≡CHCl + CCl2 → Cl2HC-CHCl2
2. Addition of halogen halides or halogen acids : Halogen halides add to alkynes, their order of reactivity being HI > HBr > HCI > HF . These additions occur in accordance with Markovnikov’s rule from first vinyl halides and then alkyliedene halides.
HC≡CH + HCl → CH2=CHCl + HCl → CH3CHCl2
When acetylene is passed through dil. HCl at 338 K in presence of Hg2+ ions as catalyst, only vinyl chloride is formed.
HC≡CH + HCl → CH2=CHCl
With hydrogen bromide, first a vinyl bromide and then an alkylidene dibromide is formed.
HC≡CH + HBr → CH2=CHBr + HBr → CH3CHBr2
CH3-C≡CH + HBr → BrCH3C=CH2 + HBr → (CH3)2CBr2
In presence of peroxides, anti- Markovnikov’s addition of HBr occurs.
CH3C≡CH → CH3CH=CHBr in presence of HBr and RCOOR
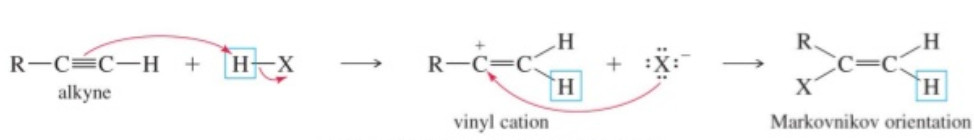
Since carbocation (I) is stablized by + R-effect of the X atom while carbocation (II) is destabilized by – I effect of X, therefore, reaction occurs through the more stable carbocation (I) forming 1, 2-dihaloethane.
HC≡CH + HOCl → [Cl-CH=CH-OH] → [Cl2CH=CH(OH)2] → Cl2CH-CHO
CH3-C≡CH + HOCl → [CH3-COH=CHCl] → [CH3-C(OH)2-CCl2] → CH3-CO-CHCl2
4. Addition of H2O – Hydration of alkynes :Alkynes cannot be hydrated as easily as alkenes because of their lower reactivity towards electrophilic addition reactions. Further, dilute H2SO4 as catalyst, hydration occurs readily. Under these conditions, H2O adds to the triple bond to form an enol which readily tautomerises to the corresponding carbonyl compound.
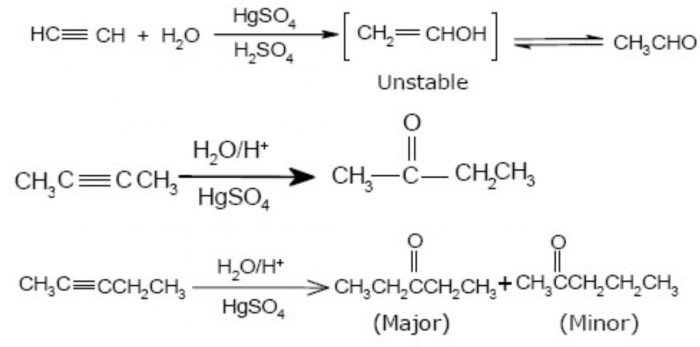
Unsymmetrical terminal alkynes, addition occurs in accordance with Markovnikov’s rule. If the unsymmetrical alkyne is non-terminal, a mixture of two isomeric ketones is obtained in which the methyl ketone predominates.
HC≡CH + CH3COOH → H2C=CH-OCOCH3 → CH3-CH(OCOCH3)2
Vinyl acetate is used for manufacture of vinyl resin. Ethylidene diacetate, when heated rapidly to 573 – 673 K, gives acetic anhydride and acetaldehyde.
CH3-C-(OCOCH3)2 → (CH3COO)2O
6. Addition of hydrogen cyanide : Acetylene adds on hydrogen cyanide in presence of Ba(CN)2 or CuCl in HCl as catalyst or acrylonitrile.
HC≡CH + HCN → CH2=CH-CN in presence of Ba(CN)2 or CuCl/HCl
Nucleophilic Addition Reaction
HC≡CH + CH3OH → CH2=CH-OCH3
Methyl vinyl ether is used for making polyvinyl ether plastics.
Reduction of Alkynes
Addition of dihydrogen to alkynes in presence of nickel at 523 – 573 gives alkanes.
HC≡CH + H2 → CH2=CH2 + H2 → CH3-CH3 in presence of Ni catalyst.
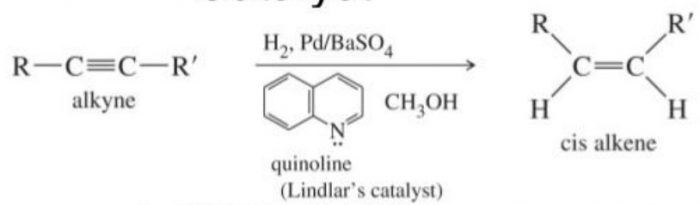
It is possible to stop the reduction at the alkene stage by using specific catalysts such as Lindlar’s catalyst i.e. Pd supported over CaCO3 or BaSO4 and partially poisoned by addition of sulphur or quinoline.
Catalytic reduction of alkynes with dihydrogen in presence of Lindlar’s catalyst always gives cis- alkenes.
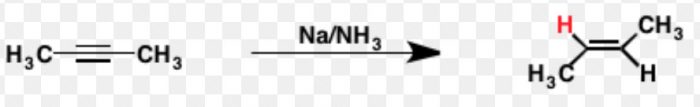
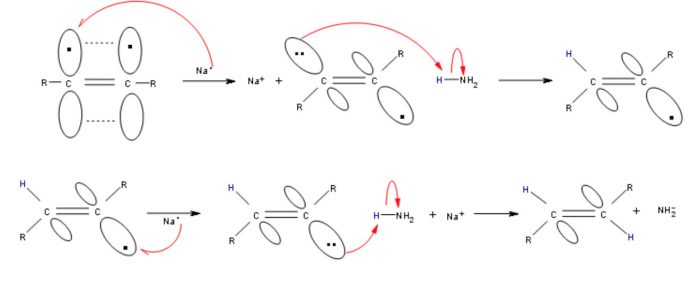
Chemical reduction i.e. Birch reduction of non-terminal alkynes with Na or Li in Liquid NH3 at 196-200 K gives trans-alkenes.
Oxidation Reaction of Alkynes
1. Oxidation with air or oxygen, Combustion
Acetylene burns with a luminous yellow sooty flame due to the presence of higher carbon content than hydrogen. However, with air or oxygen under high pressure, acetylene burns with a blue flame (oxyacetylene flame) producing high temperature of the order of 3000 K which is used for cutting and welding of metals.
2. Oxidation with cold dilute potassium permanganate
In case of terminal alkynes, ≡CH part is oxidised to -COOH group while in case of non terminal alkynes, ≡CR part is oxidised to R-C=O group.
CH3-C≡CH + 3[O] → CH3COOH + CO2 in presence of KMnO4 , H2O at 288-303K
CH3-C≡C-CH3 + 2 [O] → CH3COCOCH3 in presence of KMnO4 , H2O at 288-303K
The pink colour of the KMnO4 solution is discharged and a brown precipitation of manganese dioxide is obtained. This reaction is therefore used as test for unsaturation under the name Baeyer’s test.
3. Oxidation with hot KMnO4 solution
HC≡CH + 4 [O] → HOOC-COOH +[O] → 2CO2 + H2O in presence of KMnO4,KOH, 373-383 K
CH3-C≡CH +4[O] → CH3COOH + CO2 in presence of KMnO4,KOH, 373-383 K
Non-terminal alkynes on oxidation with hot KMnO4 solution give only carboxylic acids.
CH3-C≡CH + 4[O] → 2CH3COOH
CH3-CH2-C≡CH3 + 4[O] → CH3CH2COOH + HOOCCH3
Thus, by identifying the products formed during alkaline KMnO
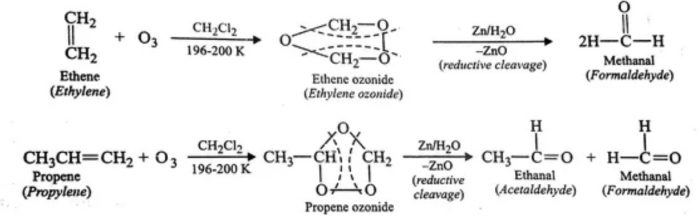
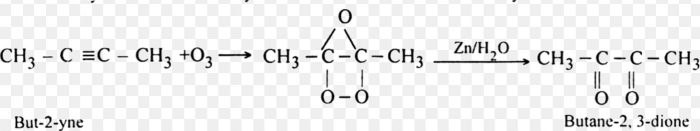
4. Oxidation with ozone
Alkynes react with ozone in presence of some inert solvents such as CH2Cl2, CHCl3 or CCl4 at low temperature (196- 200 K) to form ozonides. These ozonides on decomposition with Zn dust and water or H2/Pd (reductive cleavage) give 1, 2-dicarbonyl compounds.
5. Polymerization Reactions of Alkynes
(a) Linear Polymerization In this type of polymerization, two or more molecules of ethyne combine to form products which have linear structures.
(i) In presence of CuCl/NH4Cl, acetylene first gives vinylacetylene and then divinylacetylene.
2HC≡CH → HC≡CH=CH2 + HC≡CH → H2C=CH-C≡C-CH=CH2
Vinylacetylene is widely used in the manufacture of chloroprene which is the starting material for the synthetic rubber neoprene.
CH2=CH-C≡CH + HCl → CH2=CH-CCl =CH2
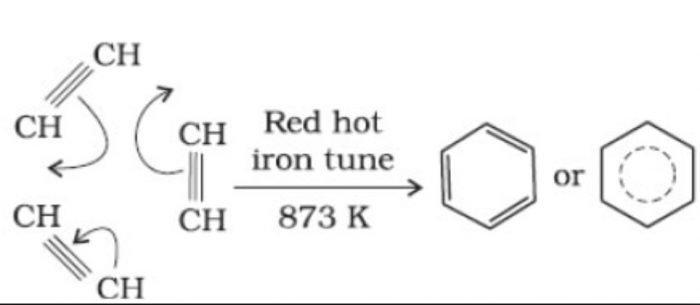
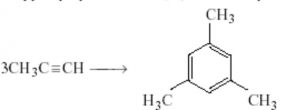
(b) Cyclic polymerization : In this type of polymerization, three or more molecules of an alkyne combine together to form products which have ring structures.
(i) When ethyne is passed through red hot iron tube at 873 K, it trimerises to give benzene.

6. Isomerism of alkynes
CH3C≡CCH3 → CH3CH2C≡CH NaNH2 in inert solvent, heat
CH3CH2C≡CH → CH3C≡CCH3 NaNH2 in inert solvent, heat
Uses of Alkynes
(i) Acetylene and its derivatives are widely used in synthetic organic chemistry for synthesis of cis and trans-alkenes, methyl ketones etc.
(ii) Oxyacetylene flame is used for cutting and welding of metals.
(iii) Acetylene is used as illuminant in hawker’s lamp and in light houses.
(iv) Acetylene is used for ripening of fruits and vegetables.
(vi) Acetylene is used for manufacturing of ethyl alcohol, acetaldehyde, acetic acid, vinyl plastics, synthetic rubbers such as Buna-N and synthetic fibres such as Orlon.
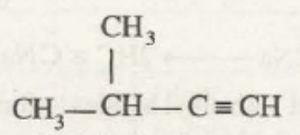

I want brain tree for chemical properties of alkynes
Thank You Very Much!
they are easy to understand thanks I need more of your note in organic chemistry