Nomenclature means the system of naming of organic compounds. In case of aliphatic compounds, two system of nomenclature generally used are:
(1) Trivial or common system
(2) IUPAC system
Contents
Trivial or Common System
Organic compounds were named after the source from which they were first isolated.
For example : Urea got its name since the compound was first obtained from the urine of mammals.
Methyl alcohol was called wood spirit since it could be obtained by the destructive distillation of wood.
Acetic Acid got its name from the acetum since it is present in vinegar.
Formic acid was derived from formicus since it could be obtained by the destructive distillation of red ants.
Citric acid is named so because it is found in citrus fruits.
Common or Trivial names of some organic compounds:
| Compound | Common Name |
| CH4 | Methane |
| C2H2 | Acetylene |
| CH3CH2CH2CH3 | Butane |
| (CH3)2CHCH3 | Isobutane |
| (CH3)4C | Neopentane |
| CHCl3 | Chloroform |
| CH3CH2OCH3 | Ethyl Methyl ether |
| HCHO | Formaldehyde |
| (CH3)2CO | Acetone |
| CH3COOH | Acetic acid |
| CH3CONH2 | Acetamide |
| CH3CN | Acetonitrile |
| C6H6 | Benzene |
| C6H5OH | Phenol |
| C6H5OCH3 | Anisole |
| C6H5NH2 | Aniline |
IUPAC system
To systematize the nomenclature of organic compounds, IUPAC system of nomenclature was first introduced in 1947.
General Rules for IUPAC Nomenclature of Organic Compounds
The most important feature of this system is that any given molecular structure has only one IUPAC name and any given IUPAC name denotes only one molecular structure.
The IUPAC name of any organic compound essentially consist of three parts ,i.e.
(1) word root
(2) suffix
(3) prefix
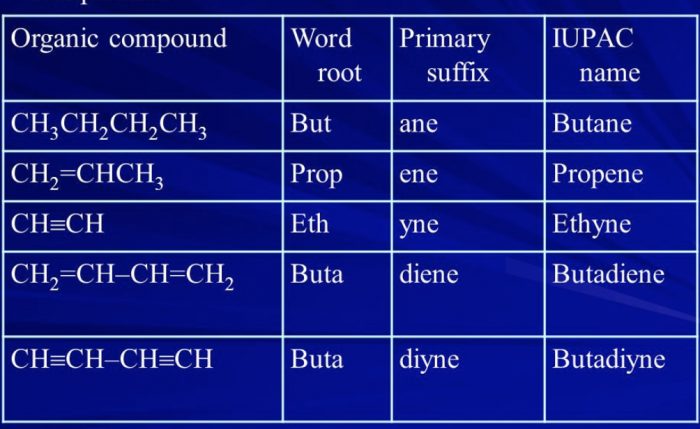
Word root
It denotes the number of carbon atoms present in the parent chain (the longest possible continuous chain of carbon atoms including the functional group and multiple bonds) of the organic molecule.
| Chain Length | Word Root |
| C1 | Meth |
| C2 | Eth |
| C3 | Prop |
| C4 | But |
| C5 | Pent |
| C6 | Hex |
| C7 | Hept |
| C8 | Oct |
| C9 | Non |
| C10 | Dec |
| C11 | Undec |
| C12 | Dodec |
Suffix
There are two types of suffix :
(1) Primary suffix
A primary suffix is always added to the word root to indicate whether the carbon chain is saturated or unsaturated.
The three basic primary suffixes are :
| Type of carbon chain | Primary suffix | General name |
| Saturated | -ane | Alkane |
| Unsaturated with one double bond | -ene | Alkene |
| Unsaturated with one triple bond | -yne | Alkyne |
If the parent chain contains two, three, four or more double or triple bonds ,numerical prefixes such as di, tri, tetra are added to the prefect primary.
| Type Of carbon chain | Primary Suffix | General name |
| Unsaturated with two double bonds | -diene | Alkadiene |
| Unsaturated with two triple bonds | -diyne | Alkadiyne |
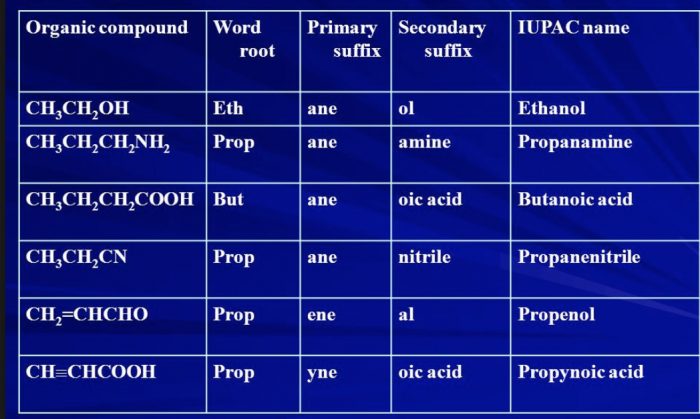
(2) Secondary suffix
A secondary suffix is then added to the primary suffix to indicate the nature of the functional group present in the organic compound.
| Class of Organic compound | Functional Group | Secondary Suffix |
| Alcohols | -OH | -ol |
| Aldehydes | -CHO | -al |
| Ketones | -CO | -one |
| carboxylic acid | -COOH | -oic acid |
| Acid amides | -CONH2 | -amide |
| Acid chloride | -COCl | -oyl chloride |
| Esters | -COOR | alkyl oate |
| Nitriles | -CN | nitrile |
| Thiols | -SH | thiol |
| Amines | -NH2 | amine |
While adding the secondary suffix to primary suffix ,the terminal e of the primary suffix is dropped if the secondary suffix begins with a vowel but is retained if the secondary suffix begins with a consonant.
(1) Locants are placed immediately before the part of the name to which they relate.
For Example : But-2-ene , Propan-2-ol
(2) The locant 1 is often omitted when there is no ambiguity.
CH3CH2CH2NH2 Propane-1-amine is often named as Propamine.
ClCH2CH2OH 2-Chloroethan-1-ol is often named as 2-Chloroethanol
CH3CH2CH2CHO Butan-1-al is often named as 2-Chloroethanol.
Prefix
There are two types of prefixes:
(1) Primary Prefix
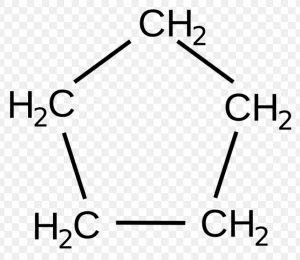
A primary prefix is used simply to distinguish cyclic from acyclic compounds.
For example : In case of carbocyclic compounds, a primary prefix, cyclo is used immediately before the word root.
Cyclopentane
Cyclo(Primary prefix) + Pent (word root) + ane( Primary suffix)
(2) secondary prefix
Certain groups are not considered as functional groups but instead are treated as substituents. These are called secondary prefixes and are added immediately before the word roots in alphabetical order to denote the side chain or substituent groups.
The secondary prefixes for some groups which are always treated as substituent group are given below:
| Substituent Group | Secondary group | Substituent Group | Secondary Prefix |
| -F | Fluoro | OCH3(OMe) | Methoxy |
| -Cl | Chloro | -OC2H5(-OEt) | Ethoxy |
| -Br | Bromo | -CH3(-Me) | Methyl |
| -I | Iodo | -C2H5(-Et) | Ethyl |
| -NO2 | Nitro | -CH2CH2CH3(n-Pr) | n-Propyl |
| |
Diazo | -C(CH3)3 | tert-Butyl |
| —NO | Nitroso | -CH(CH3)2(-iPr) | Isopropyl |
IUPAC name of an organic compound consist of:
Secondary prefix + Primary prefix + Primary suffix + Secondary Suffix
| Organic compound | Secondary Prefix | Word Root | Primary Suffix | IUPAC name |
| CH3CH2Br | Bromo | eth | ane | Bromoethane |
| CH3NO2 | Nitro | meth | ane | Nitromethane |
| C2H5OC2H5 | Ethoxy | eth | ane | Ethoxyethane |
This page very helpful for my study
fine but can be much better than it….
Good contents for study……
I want Question for naming tha compound
This content very helpful for my study
Good page for understanding more about organic chemistry
This Content is very useful for self Study……..
Thank you very much!!! It helped me a lot in my studies…
This notes are useful but not explain in simple language
Thank you so much .this notes is very easy to understand and l like this note
Helpful for me thanks…
great content fabulous.
it is very helpful for my self study
Thank you this notes are very helpful for me
Very nice and helpful too
Well explained
One day before exam it helped me a lot
We understood a lot from this madam shilpi nagpal
It has a great content with very precise pinpoints required for a quick recap. It helped me a lot.
It is great content with all examples and with formulas also it very good for my BIPC course
Thank you so much .
They contain are very useful for me.
They are basic information but enough.
Thank you so much
Thanks thanks thanks……….
very informative and healthy content. if it contains more question on IUPAC this will be much more vital.
Thanks it helped me a lot
Good content before exam revision
It is a proper and nice content about Nomenclature.
It’s very helpful