Contents
Carbanions
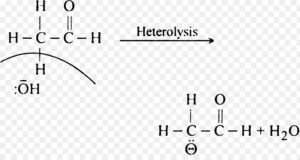
Chemical species bearing a negative charge on carbon and possessing eight electrons in its valence shell are called carbanions.
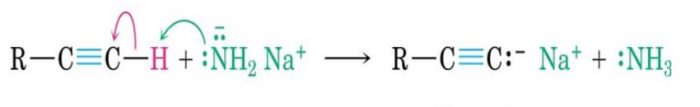
These are produced by heterolytic cleavage of covalent bonds in which the shared pair of electrons remains with the carbon atom.
Classification of Carbanion
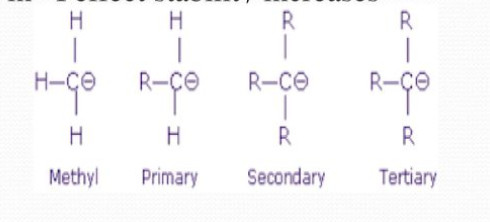
Carbanions are also classified as primary (1°), secondary (2°) and tertiary (3°) according as the negative charge is present on a primary, secondary and tertiary carbon atom.
Stability of Carbanions
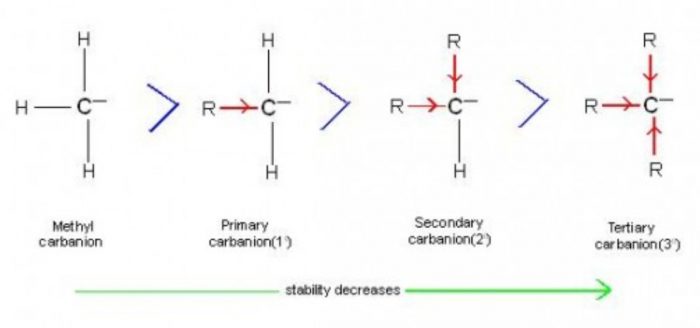
The stability of carbanions follow the order : CH3‾ >1°>2°>3°
Inductive effect : An alkyl group has +I effect. When an alkyl group is attached to a negatively charged carbon atom of the carbanions, it tends to release electrons towards the carbon .In doing so, it increases the intensity of the negative charge on the carbon and thus destabilizes the carbanion.
More the number of alkyl group on the carbon atom carrying the negative charge, more would be the intensity of the negative charge on the carbon atom and hence less stable is the carbanion.
Stability decreases in the order: CH3‾ >1°>2°>3°
Resonance Effect
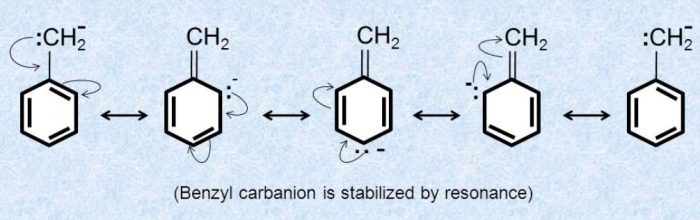
Allyl and benzyl carbanions are stabilized by resonance.
More the number of phenyl group, greater is the stability
The presence of electron withdrawing group such as -NO2, -CN, -COOR,-Cl in the benzene ring tend to disperse the negative charge and hence increases the stability of the carbanion while the presence of electron donating group such as -CH3, -OCH3, -OH intend to intensify the negative charge and hence decreases the stability of carbanion.
s-character
Stability of the carbanion increases with the increase in s-character of the carbon carrying negative charge.
Reactivity of Carbanions
The reactivity of carbanions is reverse of the stability i.e. 3° > 2° >1° > CH3‾
Orbital structure of Carbanion
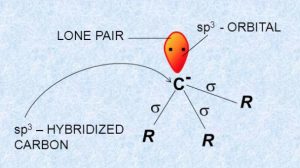
The structure of alkyl carbanion is usually pyramidal.
The carbon atom carrying the negative charge is sp3 hybridized.
Three of the four sp3 hybridized orbitals form three σ-bonds with monovalent atoms or groups while the fourth sp3 -orbital contain lone pair of electrons.
The carbanions which are stabilized by resonance are planar.In these carbanions, the carbon atom carrying the negative charge is sp2 hybridized.

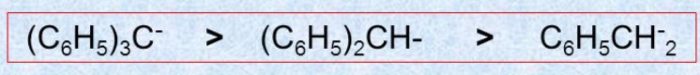

Nice
thank u so much its so infomative in easy way
hi shilpi nagpal ,
the above concept carbanion you have explained very nicely. i like it
Super mam……
Nice explanation… In simple manner
Thanks a lot!!!! It is so helpful as I study on my own.
concepts were easily explained and it has everything important for my b.sc. exam. again thanks a lot