When acetic acid is dissolved in water, it dissociates partly into H+ and H3O+ and CH3COO‾ ions as:
CH3COOH + H2O ![]() CH3COO‾ + H3O+
CH3COO‾ + H3O+
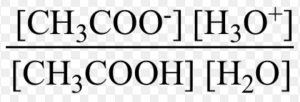
In dilute solution , concentration of water is constant. The product of K and constant ![]()
The product of K and ![]() is denoted by Ka, the ionization constant or dissociation constant of the acid.
is denoted by Ka, the ionization constant or dissociation constant of the acid.
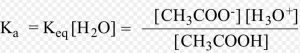
If C represents the initial concentration of the acid in moles L-1
and α , the degree of dissociation , then equilibrium concentration of the ions ( H3O+ and CH3COO‾ ) is equal to Cα and that of the undissociated acetic acid = C ( 1- α ) i.e. we have
In case of weak electrolyte, The value of α is very small and can be neglected in comparison to 1 i.e. 1-α =1.Hence we get
α = √ Ka / C
If V is the volume of the solution in litres containing 1 mole of the electrolyte , C = 1/ V.Hence, we have,
α = √ Ka × V
For a weak base like NH4OH we have
α = √ Kb / C
α = √ Kb × V
For a weak electrolyte , the degree of ionisation is inversely proportional to the square root of molar concentration or directly proportional to the square root of volume containing one mole of solute.This is called Ostwald’s dilution law.
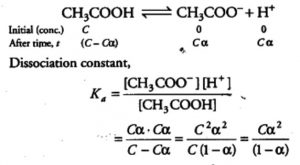
Congrats Mrs Shilpi Nagpal, for preparing a very simple text to understand the ionization of weak electrolytes. Many topics also need your methodology to understand them. I do not know whether you could prefer to write simple text to understand the friccohesity.
This information helped me a lot for my assignments of college.Thanks for providing such good understandable method of Ostwalds dillution law.
Very nice………………… Thanks