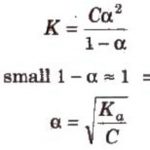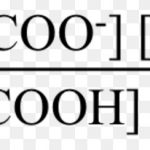According to Arrhenius concept, an acid is defined as a substance which when dissolved in water gives H+ and a base is defined as a substance which when dissolved in water give OH‾ ions. Greater the number of H+ ions produced in the aqueous solution, the stronger is the acid. Greater the number of OH‾ ions produced in the aqueous solution, the stronger is the base. As … [Read more...] about Strengths of Acids And Bases
ka
Ionisation of Weak Electrolytes
When acetic acid is dissolved in water, it dissociates partly into H+ and H3O+ and CH3COO‾ ions as: CH3COOH + H2O CH3COO‾ + H3O+ In dilute solution , concentration of water is constant. The product of K and constant The product of K and is denoted by Ka, the ionization constant or dissociation constant of the acid. If C represents the initial … [Read more...] about Ionisation of Weak Electrolytes