This theory was put forward by Heitler and London in 1927 and was further developed by Pauling and others.
In terms of energy
When the two atoms are far apart from each other there is no interaction between them.
When they come closer to each other ,the new forces come into operation.
These forces are of two types:
1)The forces of repulsion between the nuclei of these combining atoms and between the electrons of these atoms. These forces tends to increase the energy of the system.
2)The forces of attraction between the nucleus of one atom and the electrons of other atom. These forces tend to decrease the energy of the system.
If in a system, these new forces can decrease the energy ,then possibility of chemical bonding exist and if these forces lead to increase in energy, the chemical bonding is not possible.
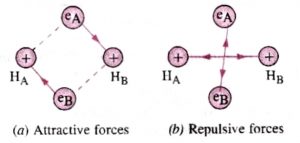
If we have two hydrogen atoms A and B kept at infinite distance from each other. There will be no interaction between them. But as they begin to come closer, the following new forces will start operating:
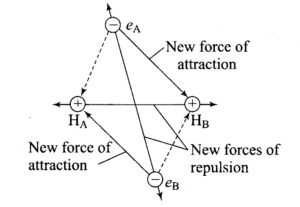
1)Force of attraction between nucleus of A and electron of nucleus B.
2)Force of attraction between nucleus of B and electron of A.
3)Force of repulsion between electrons of the atoms.
4)Force of repulsion between nuclei of the two atoms.
In hydrogen ,the magnitude of the attractive forces is more than that of repulsive forces. As a result, the potential energy of the system decreases and a molecule of hydrogen is formed .
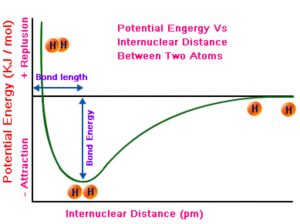
As the atoms start coming closer to each other from infinite distance, they start interacting with each other and the system starts losing its energy as the forces of attraction exceeds the forces of repulsion. But at a certain equilibrium distance ,the forces of repulsion are just balanced by the force of attraction and the energy of the system become minimum. The two hydrogen atoms are said to be bonded together to form a stable system i.e. a molecule.
The distance between the two nuclei is called Bond length.
If we want to break the bond ,i.e. to separate the atoms, we have to supply the same amount of energy.
A stronger bond is that which requires greater energy for the separation of atoms.
In the formation of a strong bond, more energy should be released by the system. Lesser the amount of energy liberated, weaker will be bond formed and larger is the amount of energy liberated, stronger will be the bond formed.
The energy required to break one mole of bonds of the same kind is known as bond energy or bond dissociation energy.
Non-formation of helium molecule
When the two atoms start moving closer to each other, four new forces of attraction and 5 new force of repulsion come into play. The attractive forces are between the two nuclei and the 4 electrons of the two atoms while out of the repulsive forces ,one is between nuclei of the two atoms and the remaining four are among the electrons.
As a result of these interaction ,repulsive forces dominate over the attractive forces and so the energy of the system increases. Hence, no chemical bond is possible.
Energetically ,the formation of helium molecule is not possible because there is an increase in the potential energy of the system when two Helium atoms approach each other.
Orbital overlap concept
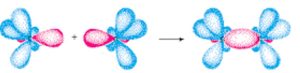
A covalent bond is formed by the partial overlap of the two half filled atomic orbitals containing electrons with opposite spins.
The partial overlap means that a part of the electron cloud of each of the two half filled atomic orbitals becomes common. The probability of finding the electrons in the region of overlap is much more than at other places.
The 2 electrons although keep on exchanging position between the two atoms but are present for maximum time in the region of overlap and hence are attracted to both the nuclei simultaneously there by forming a bond between the two atoms.
The strength of the bond depends upon the extent of overlapping. Greater the overlapping ,stronger is the bond formed.
Formation of hydrogen molecule
When two hydrogen atoms having electrons with opposite spins come close to each other, their s orbitals overlap with each other resulting in the union of the two atoms to form a molecules.
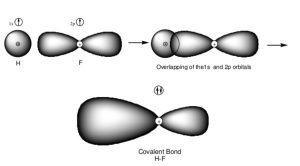
Formation of HF molecule
When one atom of fluorine having 1 unpaired electrons in its p orbital come closer to the hydrogen atom with electron of opposite Spin in its 1s orbital ,then the two half filled orbitals overlap each other and chemical bond is formed between the two atoms.
Formation of fluorine molecule
When an atom of fluorine approaches another atom of fluorine having an electron of opposite spin in 2pz orbital, the half filled orbitals overlap each other resulting in the formation of fluorine molecule.
Existence of only H2 and non- existence of species like H3 , H4
A hydrogen atom contains only one half filled atomic orbitals( 1s) which can overlap with half filled atomic orbital of another hydrogen atom only, forming H2 .No more half filled atomic orbital is available and so no more bond can be formed .
A helium atom contains fully filled atomic orbitals(1s2) which cannot overlap with the 1s orbital of another helium atom because only half filled atomic orbitals can overlap with each other.

thanks for your note
Very useful
nice
Very helpful notes thanks
Thank you so much mam…
This is very helpful…