A group of atoms existing together as one species and having characteristic properties is called a molecule.
This force which holds the atoms together within a molecule is called a covalent bond.
Noble gases neither combine chemically with any other element nor among themselves i.e. they are chemically inert.
Noble gases are inactive or stable because they have 8 electrons in the outermost shell or 2 electrons in case of helium.
The atoms of different elements combine with each other in order to complete their respective octets or duplet in case of hydrogen, Lithium and beryllium to attain stable nearest noble gas configuration.
Atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. This can occur in two ways:
1) By complete transference of one or more electrons from one atom to another. Chemical bond formed is called as electrovalent Bond or ionic bond.
2) By sharing of electrons
This can occur in two ways:
a) When the shared electrons are contributed by the two combining atoms equally ,the bond formed is called covalent bond.
b) When these electrons are contributed entirely by one of the atoms but shared by both ,the bond form is known as co-ordinate bond or dative bond.
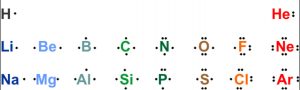
Lewis symbols
The outer shell electrons are known as valence electrons.
G.N. Lewis introduced simple symbols to denote the valence electrons in an atom.
The outer shell electrons are shown as dots surrounding the symbol of the atom.
These symbols are known as Lewis symbols are electron dot symbols.
Significance
The number of dots around the symbol give the number of electrons present in the outermost shell. This number of electrons helps to calculate the common valency of the element.
The common valency of the element is either equal to the number of dots in the Lewis structure or 8 minus the number of dots.
Li, Be, B and C have valency 1 ,2, 3 and 4 i.e. equal to the number of dots.
Valencies of N, O, F and Ne are 3 ,2, 1 and 0 i.e. 8 minus the number of dots.
Thanks man for knowledge.
Mam i told u already can you share your PDF of these notes i will be highly thankful to u…. Plzz. Provide pdf