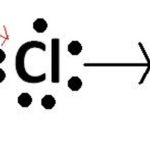When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond. On losing an electron, an atom becomes positively charged … [Read more...] about Ionic Bond
ionic bond
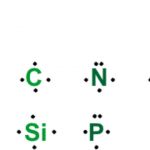
Lewis Symbols
A group of atoms existing together as one species and having characteristic properties is called a molecule. This force which holds the atoms together within a molecule is called a covalent bond. Noble gases neither combine chemically with any other element nor among themselves i.e. they are chemically inert. Noble gases are inactive or stable because they have 8 … [Read more...] about Lewis Symbols
Ionic Bond
Question 1 Define the term ionic bond? Question 2 Explain the formation of sodium chloride by electron transfer? Question 3 Give few properties of ionic compounds? Question 4 Why ionic compounds do not conduct electricity in solid state? Ionic Bond Ionic bond is formed by transfer of electrons from one atom to another. In this one atom can donate electrons to achieve … [Read more...] about Ionic Bond