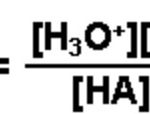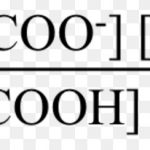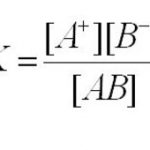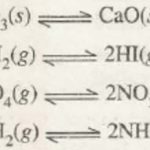Acids like HCl, H2SO4 ,HNO3 when dissolved in water dissociates almost completely thus producing a large number of H+ ions. These acids are called strong acids. Acids like CH3COOH , HF, H2CO3, H3PO4 dissociates only to a small extent in the aqueous solution giving small amount of H+ ions and hence are called weak acids. Bases like NaOH , KOH dissociate almost completely … [Read more...] about Dissociation or Ionization of Acids And Bases
Class 11
Ionisation of Weak Electrolytes
When acetic acid is dissolved in water, it dissociates partly into H+ and H3O+ and CH3COO‾ ions as: CH3COOH + H2O CH3COO‾ + H3O+ In dilute solution , concentration of water is constant. The product of K and constant The product of K and is denoted by Ka, the ionization constant or dissociation constant of the acid. If C represents the initial … [Read more...] about Ionisation of Weak Electrolytes
Concepts of Acids and Bases
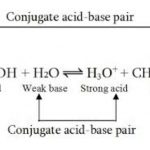
Classical concept of acids and bases Acid is a substance whose aqueous solution possessed the following characteristic properties 1) conduct electricity 2) reacts with active metals like zinc ,magnesium to give hydrogen 3) turns blue litmus to red 4) has a sour taste 5) whose acidic properties disappear on reaction with a base Base is a substance whose … [Read more...] about Concepts of Acids and Bases
Ionic Equilibrium
An electrolyte is defined as a compound whose aqueous solution or melt conduct electricity. A compound whose aqueous solution or melt does not conduct electricity is called a non-electrolyte. Aqueous solution of inorganic acids, bases and salts conduct electricity and hence they are electrolytes. Aqueous solution of sugar, urea do not conduct electricity and hence they … [Read more...] about Ionic Equilibrium
Equilibria in Chemical processes
A reaction in which not only the reactants react to form the products under certain conditions but also the products react to form reactants under the same conditions is called a reversible reaction. A reaction which takes place not only in forward direction but also in the backward direction under the same conditions is called reversible reaction, For … [Read more...] about Equilibria in Chemical processes