Heat of reaction or enthalpy of reaction is a term used for the heat changes accompanying any reaction.
Enthalpy of combustion
The enthalpy of combustion of a substance is defined as the heat change when 1 mole of substance is completely burnt or oxidised in oxygen.
CH4 ( g ) + 2O2 ( g ) —————-> CO2 ( g ) + 2H2O ( g ) ΔcH= -890.4 KJ mol -1
Reaction shows that 890.4KJ of heat is produced when 1 mole of methane is completely burnt.
Hence enthalpy of combustion of methane is 890.4 KJ mol-1
Standard enthalpy of combustion is the amount of heat evolved when 1 mole of the substance under standard conditions ( 298 K and 1 bar pressure) is completely burnt to form the products also under standard condition.
It is represented by ΔcH°
The standard enthalpy of combustion of butane is represented as:
ΔcH° = -2658 KJ mol-1
Calorific values of food and fuels
Fuels like cool ,kerosene oil, gasoline ,diesel oil are burnt to produce energy for the running of machines. Similarly for the working of the human machine, we eat carbohydrates, fats etc in the form of food.
The carbohydrates are first decomposed in our body by the enzyme to form glucose which then undergoes oxidation by the oxygen that we inhale to produce energy.
This oxidation reaction is called combustion of food.
Different fuels and foods produce different amount of heat on combustion.
The Calorific value of a fuel or food is the amount of heat in calories or joules produced from the complete combustion of 1 gram of fuel or the food.
1 mole of glucose i.e. 180 g glucose produce heat =2840 KJ
Calorific value of glucose = 2840 / 180 = 15.78 KJ g-1
A normal person needs about 3000 Kcal per day. The food consisting of Carbohydrates, oils, fats ,vitamins, proteins ,mineral ,salts etc which provide the necessary calories is called a balanced diet.
Enthalpy of formation
The enthalpy of formation of a substance is defined as the heat change i.e. heat evolved or absorbed when 1 mole of the substance is formed from its elements under given conditions of temperature and pressure.
It is usually represented by ΔfH.
The condition of temperature and pressure usually chosen as 298 K and 1 bar pressure. This is called standard state.
Standard enthalpy of formation of a substance is defined as the enthalpy change accompanying the formation of 1 mole of the substance in the standard state from its elements, also taken in the standard state. It is usually represented ΔfH°.
C ( s ) + O2 ( g ) —————> CO2 ( g ) ΔfH°= -393.5 KJ mol-1
When one mole of CO2 is formed from its elements, C ( s ) and O2 ( g ) , 393.5 KJ of heat is produced.
Standard enthalpy of formation of gaseous CO2 is 393.5 KJ
Knowing the standard enthalpy of formation of the different compounds involved in the chemical reaction, the standard enthalpy change of the given reaction can be calculated as:
For a general reaction,
ΔrH° = ( c ΔfH° ( C ) + d ΔfH° ( D ) ) – ( a ( ΔfH° ( A ) + b ΔfH° ( B ))
Enthalpy Of Neutralisation
The enthalpy of neutralisation of an acid by a base is defined as the heat change when one gram equivalent of the acid is neutralised by a base, the reaction being carried out in dilute aqueous solution.
When one gram equivalent of HCl is neutralized by NaOH or one gram equivalent of NaOH is neutralised by HCl, both solution being dilute and aqueous , 57.1 KJ of heat is produced.
The enthalpy of neutralisation of any strong acid with a strong base or vice versa, is always the same i.e. 57.1 KJ.This is because the strong acid, strong bases and salts that they form, are all completely ionized in dilute aqueous solution. The reaction between any strong acid and strong base.
Na+ + OH‾ + H+ + Cl‾ ———-> Na+ + Cl‾ + H2O ΔH= -57.1 KJ mol-1
H+ ( aq) + OH‾ ( aq ) ————> H2O ( l ) Δneut H = -57.1 KJ mol-1
Thus, enthalpy of neutralisation is the heat evolved for the reaction between the H+ ions given by the acid with the OH‾ ions given by the base to from one mole of H2O.
Enthalpy of solution
The enthalpy of solution of a substance in a particular solvent is defined as the enthalpy change when 1 mole of the substance is dissolved in a specific amount of the solvent.
However, if such a large volume of the solvent is taken that further addition of the solvent does not produce any more heat change, it is called enthalpy of solution at infinite dilution.
Water is usually used as a solvent and the symbol aq is used to represent it at large dilution.
The thermochemical equation for the dissociation of KCL and CuSO4may be represented as:
KCl ( s ) + aq ———-> KCl ( aq ) Δsol H = 18.6 KJ mol-1
CuSO4 ( s ) + aq ———–> CuSO4 ( aq ) Δsol H = – 66.5 KJ mol-1
The first case is endothermic and enthalpy of solution is 18.6 KJ mol-1
Second case is exothermic and enthalpy of solution is – 66.5 KJ mol-1
The salt like copper sulphate ,calcium chloride when present in the hydrated state dissolve with the absorption of heat.
CuSO4 . 5H20 + aq ——-> CuSO4 ( aq ) Δsol H = 11.7 KJ
Enthalpy of solution from lattice enthalpy and enthalpy of hydration
Step 1
Dissociation of ionic solid into free ions. The energy required for this process is called lattice energy or lattice enthalpy.
Step 2
Hydration of the ions.The energy released in this process is called hydration energy or enthalpy of hydration.
Δsol H = Δlattice H + Δhyd H
For 1 mole of NaCl
Lattice enthalpy = 788 KJ mol-1
Hydration Energy = – 784 KJ mol-1
Δsol H = 788 KJ mol– 1 – 784 KJ mol-1
Δsol H = 4 KJ mol-1
For most of the ionic compounds, Δsol H is positive.
Dissolution of most of the salt increases with increase in temperature.
If the lattice enthalpy of a salt is very high, dissolution of compound may not take place at all.
Calculation of lattice enthalpy from Born – Haber cycle
Lattice enthalpy of an ionic compound is the enthalpy change that occurs when 1 mole of the ionic compound dissociate into ions in the gaseous state.
Max born and Fritz Haber in 1919 put forward a method for the calculation of lattice enthalpy and hence for predicting the stability of the ionic compounds formed.
The method is known as Born-Haber cycle.
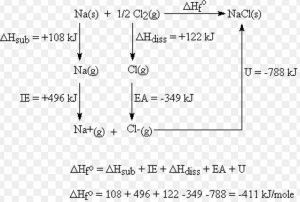
The heat of formation of NaCl ( s ) is found to be – 410 KJmol-1
1) Crystalline sodium metal is sublimed to form gaseous atom
Na ( s ) ———> Na ( g ) ΔH = Δsub H = S
2) one half mole of gaseous Cl2 molecule is dissociated to form one mole of gaseous atom
½ Cl2 ( g ) ————-> Cl ( g ) ΔH =½ Δdiss H = ½D
3)The gaseous sodium atoms are ionised to form gaseous sodium ions
Na ( g ) ———-> Na + ( g ) + e– Δ i H = I.E.
4) The gaseous chlorine atoms are converted into gaseous chloride ions by adding electrons
Cl ( g ) + e– ———> Cl‾ ( g ) Δ eg H = E.A.
5) The gaseous Na + and Cl‾ ions combine to form 1 mole of crystalline sodium chloride.
Na + ( g ) + Cl‾ ( g ) ————–> NaCl ( s ) Δlattice H = U
Greater the lattice enthalpy, more stable is ionic compounds.
Greater the lattice enthalpy of an ionic compound, less is its solubility in water.
Enthalpy of atomisation
When 1 mole of a given substance dissociates into gaseous atom ,the enthalpy change accompanying the process is called enthalpy of atomisation.
It is represented by the symbol Δa H°
Enthalpy of ionisation
When 1 mole of a covalent compound on dissolution in water splits to produce ions in the solution, the enthalpy change accompanying the process is called in therapy of ionisation.
HCl ( g ) + aq ———–> H+ ( aq ) + Cl‾ ( aq )
Enthalpy of ionization of HCl ( g ) is -75.2 KJ mol-1.
The enthalpy of ionization of a covalent compound is the same as its enthalpy of solution or enthalpy of dissociation.
Enthalpy of formation of ions
When an ionic solid is dissolved in water ,free ions are produced in the aqueous solution. For the calculation of enthalpy of formation of an ion in the aqueous solution ,enthalpy of formation of H+ ions in the aqueous solution is taken as zero.
For Ex:
½ H2 ( g ) + ½ Cl2 ( g ) ———> HCl ( g ) Δf H° = -92.8 KJ mol-1
HCl ( g ) aq —————>H+ ( aq ) + Cl‾ ( aq ) Δ diss H° = -75.2 KJ mol-1
ΔrH° = Δf H° ( H+ ( aq )) + Δf H° (Cl‾ ( aq)) – Δf H° (HCl)
-75.2 = 0 + Δf H° (Cl‾ ( aq)) – ( – 92.8 )
Δf H° (Cl‾ ( aq)) = -168 KJ mol-1
Enthalpy of hydration
The amount of enthalpy change when 1 mole of the anhydrous salt combines with the required number of moles of water so as to change into the hydrated salt, is called the enthalpy of hydration or heat of hydration.
For ex: Enthalpy of hydration of copper sulphate is -78.2 KJ mol-1
CuSO4 ( s ) + 5 H2O ———-> CuSO4 . 5H2O ( s )
Δhyd = -78.2 KJ mol-1
Enthalpy of hydrogenation
The amount of heat of hydration the enthalpy of hydration of copper sulphate is we of hydrogenation the amount of enthalpy change that take place when one more of an unsaturated organic compound is completely hydrogenated is called enthalpy of hydrogenation
Enthalpy of allotropic transformation
The enthalpy change that take place when 1 mole of one form of an allotropic modification changes to another is called is enthalpy of allotropic transformation.
C ( graphite ) ——-> C ( diamond )
S ( Monoclinic) ———–> S ( Rhombic)
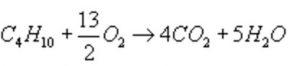
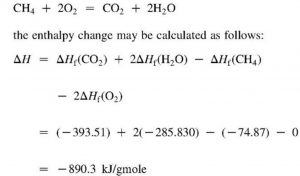
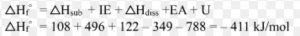
Useful notes mam
Thankyou for the notes it is useful and i also want formulae of heat of neutralisation.