Isomers which have same structural formula but have different relative arrangement or atoms or groups in space are called stereoisomers and the phenomenon is called stereoisomerism.
cis-trans isomerism is an example of stereoisomerism.
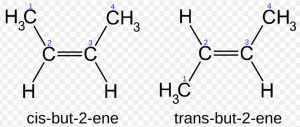
Due to π-bonding between the two carbon atoms, the rotation around carbon-carbon double bond is prohibited and hence the geometry of the atoms or groups attached to the carbon atoms gets fixed in space.
Stereoisomerism is also called geometrical isomerism.
Steric Hindrance
If two non-bonded atoms or groups in an organic molecule are held together at a distance equal to or less than the sum of their van der waal radii, then they repel each other due to spatial crowding.This repulsion is referred to as steric hindrance or steric strain or van der waal strains.
Molecules which possess steric strain are relatively less stable as compared to those having no steric strain.
For Example: Cis-but-2-ene has steric hindrance and hence is less stable as compared to trans-but-2-ene which has no steric hindrance.
As the size of the atoms around a bulky atom increases, the steric hindrance increases accordingly.
It solved my problem
Very good explanation ma’am