Quantitative analysis means to determine the percentage of each element.
Contents
Estimation of Carbon and Hydrogen
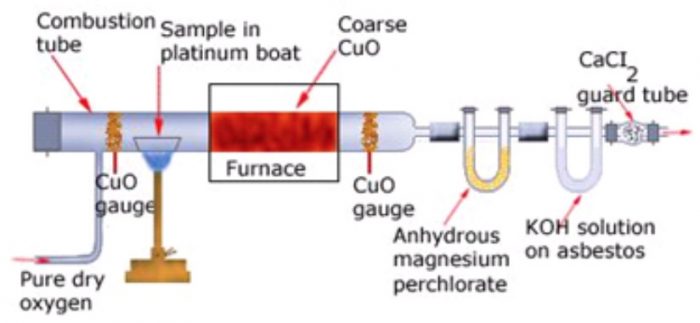
Principle. A known mass of the organic compound is heated strongly with excess of dry copper oxide in a current of dry air or oxygen (free from carbon dioxide). Under these conditions, carbon present in the organic compound is oxidized to carbon dioxide and hydrogen is oxidized to water.
C (from organic compound) + 2CuO——->CO2 + 2 Cu
2H (from organic compound) + CuO———>H2O+Cu
The water thus produced is absorbed in a U-tube containing anhydrous calcium chloride or anhydrous magnesium perchlorate while CO2 produced is absorbed in another U-tube containing a strong solution of KOH or ascarite (NaOH+ CaO). The tubes are weighed before and after the combustion. The increase in the mass of CaCl2 or Mg(ClO4)2 U-tube gives the mass of water produced while increase in the mass of KOH or ascarite U-tube gives the mass of CO2 produced.
The apparatus used for estimation of carbon and hydrogen
Calculations : Let the mass of the substance taken =w g
Mass of CO, formed = x g
Mass of water formed = y g
Percentage of Carbon
One mole of CO2 contains one gram atom of C
i.e. (12 + 2×16 ) = 44 g CO2 contain carbon= 12g
∴ x g CO2 will contain carbon = (12/44) × × g
This is the mass of carbon present in w g of the compound
%age of carbon in the compound = (12/44) × × × (100/w)
Percentage of carbon =(12/44) × (Mass of CO2 formed/Mass of substance taken)× 100
Percentage of Hydrogen
One mole of H20 contains 2 gram atoms of hydrogen
ie. (2×1 + 16)= 18 g of H20 contain hydrogen= 2 g
∴ y g H20 will contain hydrogen = 2/18 × y g
This is the mass of hydrogen present in w g of the compound.
% age of hydrogen in the compound =(2/18) × y × (100/w)
Percentage of hydrogen = (2/18 ) × (Mass of H2O formed / mass of substance taken ) × 100
Modifications under specific conditions.
Liebeg’s method is suitable in case of organic compounds containing C. H and O only. If, however, the organic compound contains one or moreof the elements like N. S and halogens also, the method is modified as under
(1) Substances containing nitrogen: Under the conditions of combustion, nitrogen, if present, in the organic compound is oxidised to oxides of nitrogen (NO, NO2) which are also absorbed in KOH solution. In such cases, a reduced copper gauze is placed near the exit end of the tube which reduces oxides of nitrogen back to N2 gas
2Cu + 2 NO ———> 2 CuO + N2
4Cu + 2 NO2 ——> 4 CuO + N2
(2) Substances containing halogens: Halogens, if present, in the organic compound are converted into cupric halides which themselves decompose to form free halogens. These halogens and volatile cupric halides also get dissolved in KOH solution. In such cases, a roll of bright silver gauze is placed near the exit end of the combustion tube. Silver gauze decomposes, volatile cupric halides forming non-volatile silver halides. Halogens also combine with silver giving silver halides.
CuX2 + 2 Ag —–> 2 AgX + Cu
4Cu + 2 NO2 ——–>4 CuO + N2
(3) Substances containing sulphur or sulphur and halogens:
Sulphur if present in the organic compound is oxidised to SO2 which will also be absorbed in KOH solution.In such cases, either an additional layer of fused lead chromate is placed after the CuO layer or CuO layer is replaced by fused lead chromate layer. At high temperatures, lead chromate decomposes to give O2 which oxidises C and H of the organic compound to CO2 and H20 respectively.
PbCrO4 ——–> 4 PbO + 2 Cr2O3 + 3 O2
4 PbCrO4 + SO2 ———> 4 PbSO4 + 2 Cr2O3 + 3 O2
4 PbCrO4 + 4 X2 —–> 4 PbX2 + 2 Cr2O3 + 3 O2
Besides, S and halogens, PbCrO4 , is also useful for phosphorus containing compounds. This is because oxides of phosphorus produced during combustion also react with lead chromate to form non-volatile lead phosphate.
Methods used for estimation of Nitrogen
The two most commonly used methods for the estimation of nitrogen in an organic compound are:
(1) Dumas method
(2) Kjeldahl’s method
I. Dumas method
This method is applicable to all organic compounds containing nitrogen.
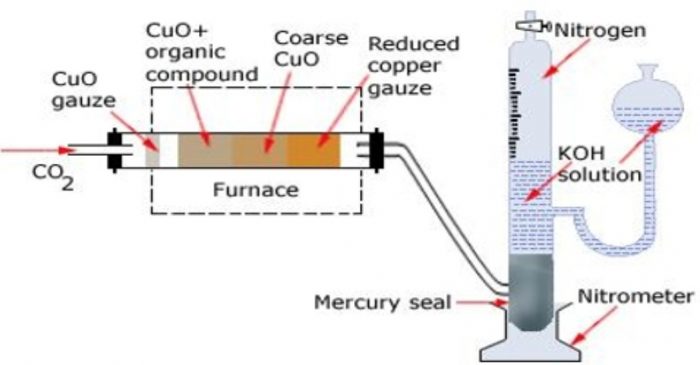
Principle: A known mass of the organic substance is heated with excess of copper oxide in an atmosphere of CO2. Carbon, hydrogen and sulphur (f present) are oxidised to CO2, H2O and SO2 respectively while nitrogen gas is set free. Any oxide of nitrogen that may be formed is reduced back to free nitrogen by passing over a hot reduced copper gauze.
C + 2 CuO —-> CO2 + 2 Cu
2H + CuO ——-> H2O + Cu
Nitrogen + CuO ——-> N2 + a small amount of oxides of nitrogen
Oxides of nitrogen + Cu ——–> CuO + N2
The nitrogen thus formed is collected over conc. KOH solution which absorbs all other gases i.e. CO2 , H2O vapours, SO2 etc. The volume of nitrogen collected is thus noted and from this the percentage of nitrogen can be calculated.
Apparatus. The apparatus used for estimation of nitrogen by Duma’s method is
It consists of three parts :
(a) carbon dioxide generator
(b) combustion tube and
(c) Schiff’s nitrometer.
Carbon dioxide generator
CO2 needed for the purpose is produced by heating sodium bicarbonate or magnesium carbonate.The gas is perfectly dried by bubbling through conc H2SO4 before passing it through the combustion tube.
Combustion tube
It is a hard glass test tube about 90 cm long and about 2 cm in diameter. It is packed with
(1) A roll of oxidised copper gauze which prevents backward diffusion of gases produced
during combustion
(2) An accurately weighed quantity of the substance mixed with excess of cupric oxide.
(3) Coarse CuO that fills nearly half of the combustion tube and
(4) A reduced copper gauze which helps to reduce any oxides of nitrogen formed during combustion back to nitrogen gas.
Schiff’s nitrometer
It consists of a long graduated tube having a reservoir and a tap at the upper end. It contains about 40% KOH solution. It also has a mercury seal at the bottom which prevent KOH solution from being sucked back into the combustion tube.
Both CO2 and H20 produced during combustion are absorbed by KOH solution white N2, is collected over it.The volume of N2 is measured after careful levelling (by making the level of KOH in the nitrometer tube and reservoir the same)
II. Kjeldahl’s method
It is largely used for the estimation of nitrogen in fertilizers, food stuffs, drugs, etc. The method is however, not applicable to compounds containing nitrogen in the ring e.g.pyridine, quinoline, etc.) and containing nitrogen directly linked to oxygen atom (e.g NO,) or another nitrogen atom ie, azo compounds.
The apparatus used for the estimation of nitrogen by Kjeldahl’s method.
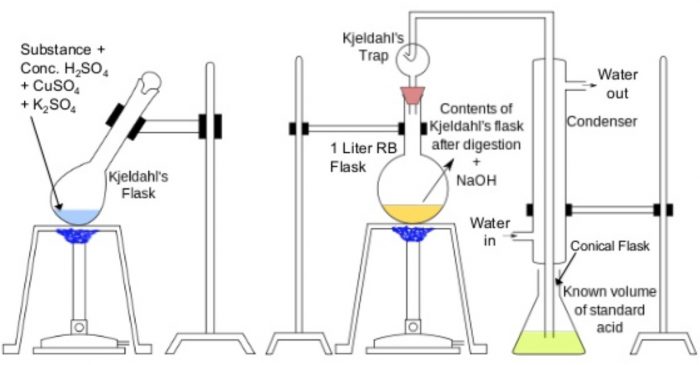
Principle: A known mass of the organic compound is heated with conc. H2SO4, in presence
of potassium sulphate and a little copper sulphate or mercury in a long-necked flask called Kjeldahl’s flask. Potassium sulphate raises the boiling point of H2SO4, and thus ensures complete reaction while copper sulphate or mercury catalyses the reaction.
As a result of heating, the nitrogen present in the organic compound is quantitatively converted into ammonium sulphate. Ammonium sulphate thus obtained is boiled with excess of NaOH solution to liberate ammonia gas which is then absorbed in a known excess of a standard acid such as H2SO4, or HCl.
N (from compound) + Conc. H2SO4 ——–>(NH4)2SO4
(NH4)2SO4 + 2 NaOH ——–> Na2SO4 + 2 H2O + 2 NH3
2 NH3 + H2SO4 ———> (NH4)2SO4
The volume of the acid left unused is found by titration against a standard alkali solution.
2NaOH + H2SO4 ——->Na2SO4 +2H2O
From this, the volume of the acid used up (or the volume of ammonia evolved) and hence the percentage of nitrogen in the organic compound can be calculated.
Calculation
Let the mass of organic substance taken =w g
Volume of H2SO4 taken =V mL of M1 molarity
and volume of NaOH used for titration of excess of H2SO4 = v mL of M1 molarity
Applying molarity equation
nb Mb Vb = na Ma Va
where nb, Mb and Vb are the acidity, molarity and volume of NaOH used while na Ma and Va are the basicity, molarity and volume of acid used.
1x v x M1 (NaOH)= 2x M1 x Va (H2SO4)
or Va =a /2
Volume of H2SO4 left unused = v/2 mL of M1 molarity
Volume of H2SO4, used up by NH3 = (V-v/2) mL of M1 H2SO4
But
(V- v/2) mL of M1 H2SO4 = 2 (V- v/2) mL of M1 NH3
Now 1000 mL of 1 M NH3 solution contain NH3 =17 g or N=14 g
2 (V-v/2) mL of M1 NH3 will contain N= (14/1000) × 2 (V-v/2) x M1 g
This is the mass of nitrogen present in w g of the substance
In general, % age of Nitrogen
%age of nitrogen
= 1.4 × Molarity of acid × Basicity of acid ×Volume of the acid used
Mass of the substance taken
If the acid used is monobasic (i.e. basicity 1), the above equation reduces to
=1.4 x Molarity of acid x Vol of acid used
Mass of the substance taken
For monobasic acids, molarity=normality
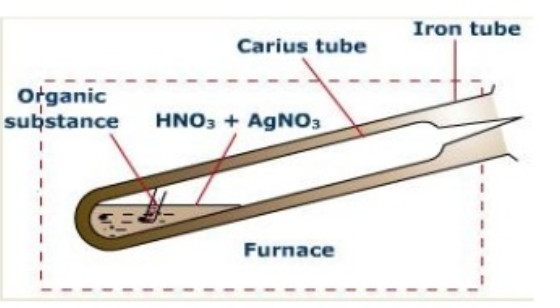
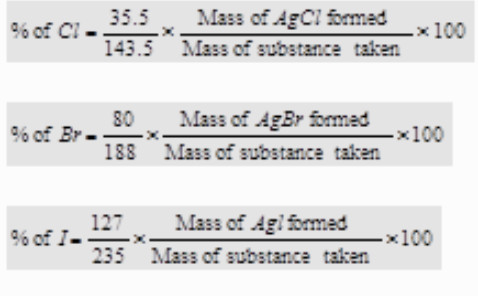
Estimation of Halogens (Carius method)
Principle: A known mass of the organic substance is heated with fuming nitric acid and a few crystals of silver nitrate in a sealed hard glass tube (about 2 cm wide 50 cm long) called Carius tube in a furnace.
Carbon and Hydrogen are oxidised to Carbon dioxide and water respectively while halogen is converted into silver halide. The precipitate of silver halide are filtered , washed ,dried and weighed.Knowing the mass of the substance taken and the mass of the precipitate formed,
the percentage of halogen is calculated as:
Calculations:
Let the mass of the substance taken=w g
and the mass of the silver halide (AgX) formed = x g
Now, 1 mole of AgX contains 1 gram atom of X ( X= Cl, Br or I)
i.e. (108 + At. mass of X) g of AgX contain X = (At. mass of X)g
∴ x g of AgX contain X =( At.mass of X × × g )/ 108 + Atomic mass of X
%age of halogen in the compound = ( At.mass of X × × g × 100 ) / ((108 + Atomic mass of X)) × x

Estimation of Sulphur (Carius method)
Principle: A known mass of the substance is heated with sodium peroxide or fuming nitric acid in a sealed tube (Carius tube). Carbon and hydrogen are oxidised to CO2 and H2O respectively while sulphur present in the compound is oxidised to sulphuric acid which is then precipitated as barium sulphate by adding excess of barium chloride solution.
C + 2O (from HNO3)——-> CO2
2H+ O (from HNO3)——-H2O
S + H2O+3O (from HNO3)——-> H2SO4
H2SO4 + BaCl2 ———> BaSO4 + 2HCl
The ppt. of BaSO4 is filtered, washed, dried and weighed. Knowing the mass of substance taken and the mass of BaSO4 ppt. formed, the percentage of sulphur can be calculated.
Calculations:
Let the mass of the substance taken w g
Mass of BaSO4 ppt. formed= x g
Now, 1 mole of BaSO4 contains 1 gram atom of S
i.e., (137 +32+ 64) 233 g of BaSO4, contain sulphur 32 g
x g of BaSO4 will contain sulphur = (32 × x g )/233
This is the mass of sulphur present in w g of the substance
% age of sulphur present in the compound= (32 × × × 100 ) / (233 × w)
%age of sulphur = (32 × Mass of BaSO4 formed × 100)/ (233 × Mass of substance taken)
Estimation of Phosphorus
Principle: A known mass of the organic compound is heated with fuming nitric acid in a sealed tube (Carius tube). Under these conditions, carbon and hydrogen are oxidised to CO2 and H2O respectively while phosphorus present in the organic compound is oxidised to phosphoric acid which is precipitated as ammonium phosphomolybdate by heating it with conc. HNO3 and then adding ammonium molybdate.
C + 2O (from HNO3)——-> CO2
2H+ O (from HNO3)——-H2O
2P + 5O (from HNO3) ——–> P2O5
P2O5+ 3 H2O ——> 2H3PO4
H3PO4+ 12 (NH4)2MoO4 + 21 HNO3 ——-> (NH4)3PO4.12MoO3 + 21 NH4NO3 + 12 H2O
The precipitates of amm. phosphomolybdate thus formed are filtered, washed, dried and weighed.
Alternatively phosphoric acid is precipitated as magnesium ammonium phosphate by adding magnesia mixture (a solution containing magnesium chloride, ammonium chloride and ammonia).
Mgcl2 + NH4Cl + H3PO4 —–> MgNH4PO4 + 3 HCl
The precipitates of magnesium ammonium phosphate are filtered, washed, dried and then ignited.
2 MgNH4PO4 ——-> Mg2P2O7 + 2 NH3 + H2O
The magnesium pyrophosphate thus formed is weighed. Knowing the mass of the organic compound taken and the mass of ammonium phosphomolybdate or magnesium pyrophosphate formed, the percentage of phosphorus can be easily calculated.
Calculation
Mass of ammonium phosphomolybdate formed = x g
Now 1 mole of (NH4)3PO4 . 12 MoO3 contains one gram atom of P i.e.
3 x (14 + 4) + 31 + 4 x 16 + 12 (96* + 3 x 16) = 1877 g of (NH4)3 PO4. 12 MO3 contain P = 31 g
Therefore, x g of (NH4)3 PO4. 12 MO3 will contain P = (31 X x)/1877 g
This is the mas of P present in w g of the compound
Therefore, % age of P = 31/1877 X x/w X 100 or
%age of P = 31/1877 X Mass of amm. phosphomolybdate /mass of substance taken X 100
Alternatively if phosphorus is estimated as Mg2P2O7.
Mass of Mg2P2O7 formed = x g
Now, 1 mole of Mg2P2O7 formed = x g
Now, 1 mole of Mg2P2O7 contains two gram atoms of P
i.e (24 X 2 + 31 X 2 + 16 X 7) = 222 g of Mg2P2O7 contain phosphorus = 62 g
Therefore x g of Mg2P2O7 will contain phosphorus = 62 x /222 g
This is the mass of phosphorus present in w g of the compound.
i.e. percentage of phosphorus present in the compound = (62 × X × 100)/ (222 × w)
Therefore percentage of phosphorus =(62 ×Mass of Mg2P2O7 formed × 100)/ (222 × Mass of substance taken)
Thanks Mam
It is very helpful for me.
Very helpful
Thanq so much
Thanku so much ✨It helps me a lot ✌️