According to Arrhenius concept, an acid is defined as a substance which when dissolved in water gives H+ and a base is defined as a substance which when dissolved in water give OH‾ ions.
Greater the number of H+ ions produced in the aqueous solution, the stronger is the acid.
Greater the number of OH‾ ions produced in the aqueous solution, the stronger is the base.
As greater is the dissociation constant of the weak acid ( Ka ) , greater is the amount of H+(aq) produced, therefore stronger is the acid.
Ka value gives a measure of the relative strength of the weak acid.
Kb value gives a measure of the relative strength of the weak base.
Suppose the weak acid is represented by HA. Suppose the initial concentration of HA is C mol/L and ∝ is the degree of dissociation.
HA + H2O H+ (aq) + A‾ (aq)
C 0 0 Initial conc
C(1-∝) C∝ C∝ Conc at equilibrium
where Ka is called dissociation constant of the acid.
If two acids of equimolar concentration are taken
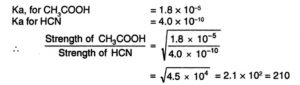
The relative strength of two acids of equimolar concentration can be compared by taking square root of the ratios of their dissociation constants.
CH3COOH is nearly 192 times stronger than HCN.
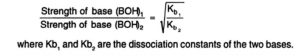
The relative strength of two weak bases of equimolar concentration can be compared by taking square root of the ratios of their dissociation constants.
Since the ionization of an acid or a base increases with dilution, the strength of the acid or base increases with dilution.
Ka and Kb are taken as dimensionless quantities because the standard state concentration of all species involved is taken as mol L-1
Polyprotic acid and polyacidic bases
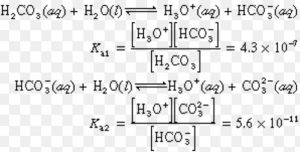
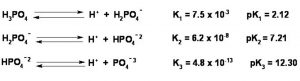
Some acids like H2SO4 , H2CO3, (COOH)2 , H3PO4 , H3AsO4 contain more than one ionizable proton.Such acids are called polybasic or polyprotic acids.They ionize in steps.
H2A (aq) H+(aq) + HA‾ (aq)
HA‾ (aq) H+(aq) + A2‾ (aq)
Ka = Ka1 × Ka2
For a tribasic acid , the overall ionization constant will be product of the ionization constant of the three steps i.e.
Ka = Ka1 × Ka2 × Ka3
Ka1 > Ka2 > Ka3
It is more difficult to remove a positively charged proton from a negative ion due to electrostatic forces.
Greater the charge on the negative ion, more difficult it becomes to remove a proton.
According to Bronsted -Lowry concept , the relative strength of two acids are compared by comparing their tendencies to donate protons. The relative strength of two bases are compared by comparing their tendencies to accept proton.
The relative strength of the two acids or the two bases involved in the two acid-base conjugate pairs can be found out if we know whether forward reaction is favoured or backward reaction is favoured.
HCl + H2O H3O+ + Cl‾
acid1 base2 acid2 base1
The forward reaction proceeds almost to completion. HCl is stronger acid than H3O+
H2O is a stronger base than Cl‾.H2O is a stronger base than Cl‾.Thus the strong acid has a weak conjugate base.
CH3COOH + H2O H3O+ + CH3COO‾
acid1 base2 acid2 base1
The backward reaction is favoured.This shows that H3O+ is stronger acid than CH3COOH.
CH3COO‾ is a stronger base than H2O . The strong acid has a weak conjugate base and strong base has the weak conjugate acid.
A strong acid has a weak conjugate base and vice versa.
Factors affecting strength of hydroacids
1) Weaker the H-A bond, more easily it dissociate to give H+ ion and hence stronger is the acid.
2) Greater the polarity of the H-A bond i.e. larger the electronegativity difference between the atoms H and A, more easily the bond breaks and greater is the acidity.
3) For elements in the same group
The bond strength dominates over the polar nature.Down the group , as size of the atoms increases, bond strength decreases and hence acidic strength increases.
HF < HCl < HBr < HI
H2O <H2S <H2Se <H2Te
4) For elements along the same period
The polarity of H-A bond decides the acidic strength.With the increase in the electronegativity of A , the polarity of the bond increases and hence the acidic strength also increases.
CH4 < NH3 <H2O <HF
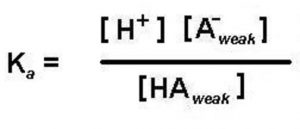
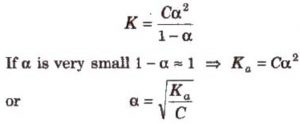

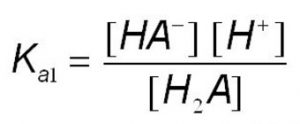
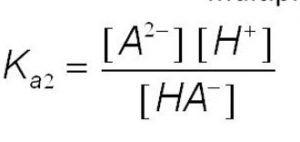
Leave a Reply