When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond.
On losing an electron, an atom becomes positively charged since the number of protons exceeds the number of electrons.
A——–> A+ + e‾
On gaining the electron, the number of electrons exceeds the number of protons and thus atom become negatively charged.
B + e‾ ——–> B‾
The oppositely charged particles attract each other by electrostatic forces of attraction. The bond formed is called electrovalent or ionic bond.
Such a type of bond is formed only when one of the atoms can easily lose electron while the other can gain electron and thus each acquires the stable electronic configuration arrangement of the nearest noble gas.
Formation of sodium chloride
Sodium( Z=2,8,1) has one electron in its outermost shell. By losing one electron of its outermost shell, it acquires the inert gas configuration and changes into sodium ion.
Chlorine( Z= 2,8,7) accepts one electron released by sodium to complete its octet and acquires stable configuration and changes into chloride ion.
The positively charged sodium ion and negatively charged chloride ions are held together by strong electrostatic force of attraction.
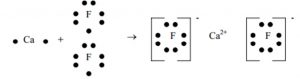
Formation of calcium chloride
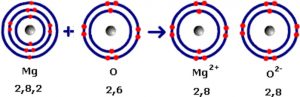
Calcium(Z=2,8,8,2) atom loses 2 electrons to attain stable noble gas configuration and gets converted into calcium ion.
Fluorine can gain one electron to acquire the stable configuration and gets converted into fluoride ion.
Calcium atom loses 2 electrons and forms calcium ions with 2 units of positive charge and two electrons are transferred to two fluorine atoms, which are converted into fluoride ions each with one unit negative charge. These oppositely charged ions are held together by strong electrostatic forces of attraction.
The number of electrons lost or gained during the formation of an electrovalent linkage is termed as
electrovalency of the element.
Sodium and Calcium loses one and two electrons and so their valencies are 1 and 2.
Chlorine and oxygen gain 1 and 2 electrons so their valencies and 1 and 2.
Coordination number of an ion may be defined as the number of oppositely charged ions present as the nearest neighbour around that ion in an ionic crystal.
Factors governing the formation of Ionic bond
The formation of ionic bond involves:
1) the formation of a positive ions by loss of electrons from one kind of atoms.
2)the formation of a negative ions by by gain of electrons from another kind of atom.
3)holding the positive and negative ions by electrostatic force of attraction.
The formation of ionic bond depends upon the following factors:
1)Ionisation enthalpy
Ionisation enthalpy of any element is the amount of energy required to remove an electron from the outermost shell of an isolated atom in gaseous phase so as to convert it into a gaseous positive ion.
a) Lesser the Ionization enthalpy, easier will be the removal of an electron i.e. formation of a positive ion and hence greater the chances of formation of an ionic bond.
b)Ionization enthalpy of alkali metals is low, hence they have more tendency to form positive ions.
c)In alkaline earth metals the formation of positive ions takes place but the ionization enthalpies are higher than those of group one element, hence the formation of positive ion is not as easy as in case of alkali metals.
Formation of positive ion of sodium is easier than that of magnesium because the energy required for the removal of second electron from it is very high hence the formation of divalent ion becomes difficult.
Lower the values of ionisation enthalpy, greater that chances of ionic bond formation.
2) Electron affinity
Electron affinity of an element is the enthalpy change that take place when an extra electron is added to an isolated atom in the gaseous phase to form a gaseous negative ion.
Higher is the electron affinity, more is the energy released and stabler will be the negative ion produced.
The probability of formation of ionic bond will be enhanced. Halogens possess high electron affinity. So the formation of their negative ions is very common.
Elements of group 16 form divalent negative ions but not so easily because the second electron affinity in case of these elements is negative i.e. energy is required to found divalent ion.
Oxygen will add up one electron to form monovalent ion which is accompanied by release of certain amount of energy. In addition of second electron to the monovalent oxygen ion, energy is required to overcome the force of repulsion exerted by the negatively charged monovalent ion to the incoming electron.
Lattice Enthalpy
The energy released when the requisite number of gaseous positive and negative ions combine to form one mole of the ionic compound is called lattice enthalpy.
The higher the value of lattice enthalpy of the resulting ionic compound, the greater will be the stability of the compound and hence greater will be the ease of its formation.
The value of lattice enthalpy depends upon two factors:
1)Charge on the ions
The higher the charge on the ions, greater is the force of attraction and hence larger is the amount of energy released.
2)Size of the ions
If the size of the ions is large, internuclear distance will be more and force of attraction will be less while in case of small ions, internuclear distance is less and so force of attraction is greater.
If lattice enthalpy + electron gain enthalpy > Ionization enthalpy, net effect will be the release of energy and hence ionic bond is formed.
Characteristics of ionic compounds
1)Physical state
These compounds usually exist in the solid state
2)Crystal structure
They exist as ions and not as molecule. These ions are arranged in a regular pattern in the three dimensional space to form lattice. The pattern of arrangement depends upon the size and charge of the ions.
In sodium chloride, each sodium ion is surrounded by 6 chloride ions and each chloride ion by 6 sodium ions, thus giving rise to a three-dimensional octahedral crystal structure.
3)High melting and boiling point
Ionic compounds possesses high melting and boiling point because ions are tightly held together by strong electrostatic forces of attraction and hence a huge amount of energy is required to break the crystal lattice.
4)Solubility
Electrovalent compounds are soluble in solvents like water which are polar in nature and have high dielectric constant. Non-polar solvents like carbon tetrachloride, benzene having low dielectric constant are not capable of dissolving ionic solids.
5)Electrical conductivity
Ionic compounds are good conductor of electricity in solution or in the molten state. In solution or molten state their ions are free to move. As the ions are charged, they are attracted towards electrodes and thus act as carriers of electric current.
6)Ionic reaction
As the oppositely charged ions combine quickly, these reactions are quite fast.
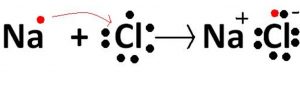
Very helpful for revision .
I appreciate your notes that are helpful.
very useful note