Whenever a molecule contains a hydrogen atom linked to a highly electronegative atom, this atom attracts the shared pair of electrons more and so this end of the molecules becomes slightly negative while the other end become slightly positive.
The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This bond is called hydrogen bond.
As a result of hydrogen bonding, a hydrogen atom links the two electronegative atoms simultaneously, one by a covalent bond and the other by a hydrogen bond.
Contents
Conditions for hydrogen bonding
1) The molecule must contain a highly electronegative atom linked to hydrogen atom. The higher the electronegativity, more is the polarization of the molecule.
2) The size of the electronegative atom should be small. The smaller the size ,the greater is the electrostatic attraction.
Examples of hydrogen bonding
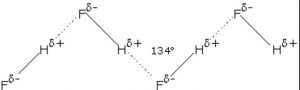
1) Hydrogen fluoride
Fluorine having the highest value of electronegativity forms the strongest hydrogen bond.
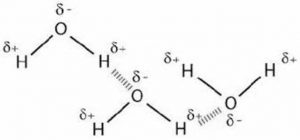
2) Water
Water molecule contains highly electronegative oxygen atom linked to hydrogen atom. Oxygen atom attract the shared pair of electrons more and this end of the molecule becomes negative whereas the hydrogen atoms become positive.
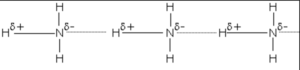
3) Ammonia
It contains highly electronegative atom nitrogen linked to hydrogen atoms.
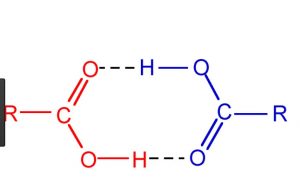
4) Alcohols and carboxylic acid
Strength of hydrogen bond
Hydrogen bond is a very weak bond. The strength of hydrogen bond is intermediate between the weak van der waals forces and the strong covalent bonds.
The dissociation energy of the hydrogen bond depends upon the attraction of the shared pair of electrons and hence on the electronegativity of the atom.
Effect or consequences of hydrogen bonding
1) Dissociation
In aqueous solution ,HF dissociates and gives the difluoride ion instead of fluoride ion. This is due to hydrogen bonding in HF. The molecules of HCl, HBr, HI do not form hydrogen bonding. This explains the non -existence of compounds like KHCl2 , KHBr2, KHI2.
2) Association
The molecules of carboxylic acids exist as dimer because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formula.
3) High melting and Boiling point
The compounds having hydrogen bonding show abnormally high melting and boiling points. The high melting and boiling point of the compound containing hydrogen bonds is due to the fact that some extra energy is needed to break these bonds.
a) The unusually high boiling point of hydrogen fluoride among the halogen acid is due to the existence of hydrogen bonding.
b) H2O is a liquid whereas H2S, H2Se and H2Te are all gases at ordinary temperature. In water ,hydrogen bonding causes association of the water molecules with the result that the boiling point of water is more than that of the other compounds. There is no such hydrogen bonding inH2S, H2Se and H2Te.
c) Ammonia has higher boiling point than PH3 because there is hydrogen bonding in NH3 but not in PH3.
d) Ethanol has higher boiling point than diethyl ether because there is hydrogen bonding in the ethanol.
e) Solubility
Lower alcohols are soluble in water because of the hydrogen bonding which can takes place between water and alcohol molecule.
f) Volatility
As the compounds involving hydrogen bonding between different molecules have higher boiling point, so they are less volatile.
g) Viscosity and surface tension
The substances which contain hydrogen bonding exist as associated molecule. So their flow becomes comparatively difficult. They have higher viscosity and high surface tension.
h) Lower density of ice than water
In case of solid ice ,the hydrogen bonding gives rise to a cage like structure of water molecules. As a matter of fact, each water molecule is linked tetrahedral to four water molecules. The molecules are not so closely packed as they are in liquid state. When ice melts ,this case like structure collapses and the molecules come closer to each other. Thus for the same mass of water, the volume decreases and hence density increases. Therefore, ice has lower density than water at 273 K. That is why ice floats.
Types of Hydrogen Bonding
Intermolecular hydrogen bonding
When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
For Ex: HF, H2O, ROH, water ,alcohol, ammonia.
Intramolecular hydrogen bonding
The hydrogen bonding which take place within a molecule itself is called intramolecular hydrogen bonding.
It takes place in compounds containing two groups such that one group contains hydrogen atom linked to an electronegative atom and the other group contains a highly electronegative atom linked to a lesser electronegative atom of the other group.
The bond is formed between the hydrogen atom of one group with the more electronegative atom of the other group.
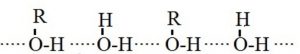
Thanks for helping and spreading education for all over the world. It’s helps a lot.
Can we download these notes ???
If yes!then how???
Thank you so much