Question 1 What is a saturated solution? Explain with example?
Question 2 What is the effect of temperature on saturated solution?
Question 3 Define the term solubility. Give example?
Also Read NCERT Solutions for Chapter 5 Separation of Substances
When we dissolve a substance in water then a solution is formed. A given quantity of water can dissolve only a certain maximum amount of substance. If we go on adding more and more of the substance in a given quantity of water then the excess substance will remain undissolved in water.
Activity
Take a beaker and put 100 ml of water in it. Add one teaspoonful of salt to water and stir it with a glass rod until the salt dissolves completely. Again add a teaspoonful of salt and stir it well. We go on adding more and more salt in water with constant stirring to dissolve it. After adding a number of spoons of salt ,we will find that some salt is left undissolved at the bottom of the beaker. This means that no more salt can be dissolved in the quantity of water which we took in the beaker. The solution is now said to be saturated.
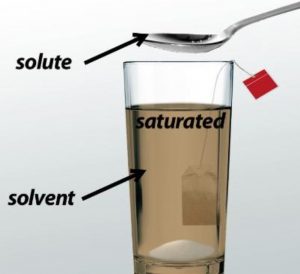
Saturated Solution
A solution in which no more substance can be dissolved at that temperature is called a saturated solution. A saturated solution contains the maximum amount of substance which can be dissolved in it at that temperature.
For ex: A maximum of 36 grams of salt can be dissolved in 100 grams of water at 20° C ,so a saturated solution of salt at 20° C contains 36 gram of salt dissolved in 100 grams of water.
Solubility
Solubility the maximum amount of a substance which can be dissolved in 100 grams of water at a given temperature is known as the solubility of that substance in water. Solubility of a substance refers to its saturated solution. Solubility of a substance depends on temperature .
The same quantity of water can dissolve different amount of different substances. The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature. Lesser amount of a substance will dissolve in a given amount of water on cooling it.
Effect of heating and cooling on saturated solution
If a saturated substance at a particular temperature is heated to a higher temperature, then the solubility of substance increases and more of substance can be dissolved in it.
If the saturated solution of a substance at a particular temperature is cooled to a lower temperature, then the solubility of the substance decreases and some of the dissolved substance will separate out in form of solid crystals.
Hot water will dissolve more substance whereas cold water will dissolve less substance.
| Notes for Chapter 5 Separation of Substances |
Very nice topic
Nicely explained. And nice notes for me as a student
Such teachers are rare.Proud of such nation builders very nicely explained concept of solubility and saturated solution.
it was so helpful
It’s so helpful thanku to google and the posting site.
Thanks a lot
as a student it is helpful