Stability Of Coordination compounds in Solution The stability of a complex in solution means the degree of association between the metal ion and the ligands involved in the state of equilibrium. The magnitude of the (stability or formation) equilibrium constant for the association express quantitatively the stability. For example: The formation of [Cu(NH3)4]2+ complex may … [Read more...] about Stability of Coordination Compounds in Solution
Class 12
Crystal Field Theory
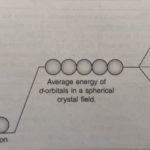
Crystal Field Theory Crystal field theory is based on the assumption that the metal ion and the ligands act as point charges and the interactions between them are purely electrostatic. In negative ligands (anions such as Cl‾, Br‾, CN‾), the interactions with metal ions are ion-ion interactions. If the ligands are neutral molecules (such as NH3, H2O, CO), the interactions … [Read more...] about Crystal Field Theory
Valence Bond Theory For Bonding In Coordination Compounds
Bonding In Coordination Compounds Valence Bond Theory For Bonding In Coordination Compounds The Main Assumptions of this Theory are listed below : (1) The central metal ion in the complex makes available a number of empty orbitals for the formation of coordination bonds with suitable ligands. The number of empty orbitals made available for this purpose is equal to … [Read more...] about Valence Bond Theory For Bonding In Coordination Compounds
Isomerism in Coordination Compounds
Isomerism Two or more compounds having the same molecular formula but different arrangement of atoms are called isomers and the phenomenon is called isomerism. Because of different arrangement of atoms, isomers differ in one or more physical or chemical properties. Isomers can be broadly classified into two major categories : (A) Structural isomers (B) … [Read more...] about Isomerism in Coordination Compounds
IUPAC Nomenclature of Coordination Compounds
IUPAC Nomenclature of Coordination Compounds Rules for Writing Formula The formula of a compound is a shorthand method used to provide basic information about the constitution of a compound in a concise and convenient manner. (1) The formula of the cation whether simple or complex is written first followed by that of the anion. (2) The coordination entity is written … [Read more...] about IUPAC Nomenclature of Coordination Compounds
![[Co(NH3)6]3+](https://classnotes.org.in/wp-content/uploads/CoNH363-150x150.jpeg)
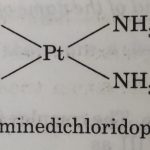
![[Pt(Cl2(NH3)2]](https://classnotes.org.in/wp-content/uploads/PtCl2NH32-150x150.jpeg)