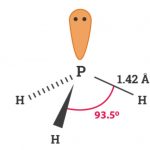
Phosphorus and Phosphine – The p-Block Elements – Class 12 Phosphorus Phosphorus is produced by heating bone ash or phosphate rock with silica (SiO2) and coke in an electric furnace at 1775 K. The reactions taking place are : 2Ca3(PO4)2 + 6SiO2 -------> 6CaSiO3 + P4O10 P4O10 +10C -------> 10 CO + P4 The vapour of phosphorus thus produced upon … [Read more...] about Phosphorus and Phosphine
The p-Block Elements
Nitric Acid
Nitric Acid – The p-Block Elements – Class 12 Nitric Acid (HNO3) The common oxoacids of nitrogen are given below : Nitric acid is a very strong oxidising agent.Nitrogen shown an oxidation state of +5 in nitric acid. Laboratory Preparation of Nitric Acid In the laboratory, nitric acid can be prepared by heating sodium or potassium nitrate with … [Read more...] about Nitric Acid
Oxides of Nitrogen
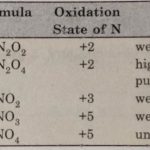
Oxides of Nitrogen – The p-Block Elements – Class 12 Oxides of Nitrogen Nitrogen combines with oxygen under different conditions to form a number of binary oxides which differ with respect to the oxidation state of the nitrogen atom. They range from N2O (oxidation state of N +1) through NO (+2), N2O3 (+3), N2O4 (+4) to N2O5(5). The tendency to form pπ- pπ multiple … [Read more...] about Oxides of Nitrogen
Dinitrogen and Ammonia
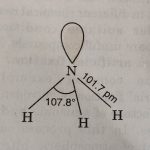
Dinitrogen and Ammonia - The p-Block Elements - Class 12 Dinitrogen 1) It is the first member of group 15 of the periodic table. 2) It has the electronic configuration 1s2 2s2 2p3 and therefore, has five electrons in its valence shell. 3) In the molecular form it exists as a diatomic molecule (N2) having triple bond between nitrogen atoms (NΞN). Therefore, it is also … [Read more...] about Dinitrogen and Ammonia
Chemical Properties of Group 15 Elements
Chemical Properties of Group 15 Elements – The p-Block Elements – Class 12 Chemical Properties of Group 15 Elements Nitrogen differs from rest of members of the group due to its (1) smaller size, (2) high electronegativity, (3) high ionisation enthalpy and (iv) non-availability of d-orbitals in the valence shell. Nitrogen has unique tendency to form pπ-pπ … [Read more...] about Chemical Properties of Group 15 Elements