Nitric Acid – The p-Block Elements – Class 12
Contents
Nitric Acid (HNO3)
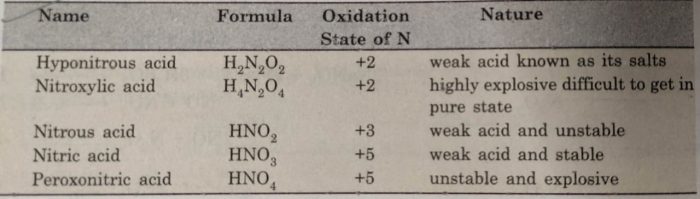
The common oxoacids of nitrogen are given below :
Nitric acid is a very strong oxidising agent.Nitrogen shown an oxidation state of +5 in nitric acid.
Laboratory Preparation of Nitric Acid
In the laboratory, nitric acid can be prepared by heating sodium or potassium nitrate with concentrated sulphuric acid to about 423-475 K.
NaNO3 + H2SO4 ——> NaHSO4 + HNO3
Anhydrous nitric acid can be obtained by distillation of concentrated aqueous nitric acid with P4010.
Manufacture of Nitric Acid
Nitric acid is commonly manufactured by Ostwald process in which it is prepared by the catalytic oxidation of ammonia by atmospheric oxygen. The reaction is carried out at about 500 K and 9 x 105 Pa (9 bar) pressure in the presence of Pt or Rh gauge as catalyst.
4NH3(g) + 502(g)——> 4NO(g) + 6H20(g) ΔH =- 90.2 kJ
Pt/Rh gauge, 500K, 9 bar
Nitric oxide thus formed combines with oxygen to form nitrogen dioxide.
2NO(g) + O2 (g) ——> 2 NO2 (g)
Nitrogen dioxide so formed, dissolves in water to give nitric acid.
3NO2 (g) + H2O(l) —–> 2HNO3(aq) + NO(g)
Dilute nitric acid is further concentrated by dehydration with concentrated sulphuric acid to get about 98% acid.
Properties of Nitric Acid
Physical Properties
1) Pure nitric acid is a colourless liquid.
2) It has boiling point 355.6 K and freezing point 231.4 K.
3) laboratory grade nitric acid contains about 68% of HNO3 by mass and has a specific gravity of 1.504.
4) The impure acid is generally yellow due to the presence of nitrogen dioxide as impurity. Nitric acid containing dissolved nitrogen dioxide is known as fuming nitric acid.
5) It has a corrosive action on skin and produces painful blisters.
Chemical Properties
(1) Acidic character: It is one of the strongest acids because it is highly ionised in aqueous solution giving hydronium and nitrate ions.
2HNO3(aq) +H2O (l) ——> H3O+ + NO3¯(aq)
It turns blue litmus red. It forms salts with alkalies, carbonates and bicarbonates.
NaOH + HNO3 —-> NaNO3 + H2O
Na2CO3 + HNO3 —-> 2NaNO3 + H2O + CO2
NaHCO3 + HNO3 —-> NaNO3 + H2O + CO2
(2) Action on metals: With the exception of gold and platinum, nitric acid attacks all metals forming a variety of products. The product depends upon the nature of metal, the concentration of acid and temperature.
(A) Metals that are more electropositive than hydrogen (Mg, Al, Mn, Zn, Fe, Pb, etc.). In this case nascent hydrogen is liberated which further reduces nitric acid.
M + 2HNO3 ——> M(NO3)2 + 2H
HNO3 + H —-> Reduction product + H2O
The principal product is NO2, with conc. HNO3, N2O with dil. HNO3, and ammonium nitrate with very dil. HNO3.
For example: Zn reacts as:
(a) Using concentrated nitric acid (forms nitrogen dioxide)
Zn + 2HNO3 —–> Zn(NO3)2 + 2H
HNO3 + H —–> NO2 + H2O] x 2
—————————————————-
Zn + 4HNO3 —-> Zn(NO3)2 + 2NO2 + 2H2O
(b) Using dilute nitric acid (forms nitrous oxide)
Zn + 2HNO3 —–> Zn(NO3)2 + 2H ] × 4
2 HNO3 + 8 H —–> NO2 + 5 H2O
—————————————————-
Zn + 10HNO3 —-> 4Zn(NO3)2 + NO2 + 5H2O
(c) Using very dilute nitric acid (forms ammonium nitrate)
Zn + 2HNO3 —–> Zn(NO3)2 + 2H ] × 4
HNO3 + 8H —–> NH3 + 3 H2O
NH3 + HNO3 ——–> NH4NO3
———————————————————-
4Zn + 10 HNO3 —–> 4Zn(NO3)2 + NH4NO3 + 3 H2O
(B) Metals which are less electropositive than hydrogen (Cu, Bi, Hg, Ag). In this case nascent hydrogen is not liberated.
HNO3 → Reduction product + H2O + [0]
Metal + (O) + HNO3→ Metal nitrate + H2O
The principal product is NO2 with conc. HNO3 and NO with dil. HNO3
For example: Cu reacts as
(a) Using concentrated nitric acid
2HNO3 ——> 2NO2 + H2O + [O]
Cu + O + 2HNO3 ——–> Cu(NO3)2 + H2O
—————————————————-
Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O
(b) Using dilute nitric acid
2HNO3 —–> 2NO + H2O + 3[O]
Cu + O + 2HNO3 –> Cu(NO3)2 + H2O] x 3
——————————————————-
3Cu + 8HNO3 ——-> Cu(NO3)2 + 2NO + 4H2O
Hg + 4HNO3 —–> Hg(NO3)2 + 2NO2 + 2H2O
6Hg + 8HNO3 —–> 3Hg2(NO3)2 + 2NO + 4H2O
Ag + 4HNO3 —–> AgNO3 + NO2 + 2H2O
3 Ag + 4 HNO3 —–> 3 AgNO3 + NO + 2H2O
(c) Action on noble metals
Noble metals like gold and platinum are not attacked by nitric acid. However, these metals are attacked by aqua regia (3 parts conc. HCl and 1 part conc. HNO3) forming their chlorides.
NaOH + HNO3 —–> NaNO3 + H2O
HNO3 + 3 HCl ——> NOCl + 2 H2O + 2 Cl
Nitrosyl chloride
Au + 3 Cl —> AuCl3
Pt + 4Cl —-> PtCl4
(3) Oxidising nature -Oxidation of non-metals and compounds.
Nitric acid behaves as a strong oxidising agent. It has a tendency to give nascent oxygen as:
2HNO3 —–> 2 NO2 + H2O + O
(conc.)
2HNO3 —–> 2 NO + H2O + 3 [O]
Therefore, nitric acid oxidises many non-metals and compounds.
(A) Oxidation of non-metals: Dilute nitric acid has no action on non-metals like carbon, sulphur, phosphorus, etc. However, concentrated nitric acid oxidises many non-metals.
For example
(1) Nitric acid oxidises sulphur to sulphuric acid
2HNO3 ——> 2NO2 + H2O+ O] x 3
1/8 S8 + H2O + 3O —–> H2SO4
——————————————————————
1/8 S8 + 6HNO3 ——–>H2SO4 + 6NO2 + 2 H2O
———————————————————
S8 + 48HNO3 ——–> 8 H2SO4 + 48NO2 + 12H2O
———————————————————-
(ii) Nitric acid oxidises carbon to carbonic acid
2HNO3 —–> 2NO2 + H2O + O] x 2
C + H2O+ 2O —-> H2CO3
————————————————–
C+4HNO3 —–> H2CO3 + 4NO2 + 2H2O
————————————————–
(iii) Nitric acid oxidises phosphorus to phosphoric acid
2HNO3 —–> 2NO2 + H2O + O] x 5
2P + 3H2O + 5O —-> 2 H3PO4
—————————————–
2P+ 10HNO3 —–> 2 H3PO4 + 10 NO2 + 2 H2O
p + 5 HNO3 —> H3PO4 + 5 NO2 + H2O
P4 + 20 HNO3 —-> H3PO3 + 20 NO2 + 4 H2O
(iv) It oxidises iodine to iodic acid.
2HNO3 ——> 2NO2 + H2O + O]× 5
I2 + H2O + 5O —> HIO3
———————————————————
I2 + 10HNO3 ——–> 2 HIO3 + 10 NO2 + 4 H2O
———————————————————
(v) Nitric acid oxidises arsenic to arsenic acid.
2HNO3 ——> 2NO2 + H2O + O] × 5
2As + 3H2O + 5O ——> 2H3AsO4
———————————————————-
2As + 10HNO3 ——> 10NO2 + 2H3AsO4 + 2H2O
As + 5HNO3 ——> 5NO2 + H3AsO4 + H2O
(B) Oxidation of compounds
Dilute as well as concentrated nitric acid oxidises a number of compounds.
(1) Nitric acid oxidises hydrogen sulphide to sulphur.
dil HNO3 :
3H2S + 2 HNO3 —–> 2 NO + 4 H2O + 3S
Conc HNO3
3H2S + 2 HNO3 —–> 2 NO2 + 4 H2O + S
(2) Nitric acid oxidises sulphur dioxide to sulphuric acid
dil HNO3
3SO2 + 2HNO3 + 2H2O —> 3 H2SO4 + 2 NO
conc. HNO3
SO2 + 2 HNO3 ——-> H2SO4 + 2 NO2
(3) Nitric acid oxidises ferrous sulphate to ferric sulphate
dil HNO3
6FeSO4 + 2HNO3 +3H2SO4——> 3Fe2(SO4)3 + 2NO + 4 H2O
conc. HNO3
2FeSO4 + 2HNO3 +3H2SO4——> 3Fe2(SO4)3 + 2NO2 + 4 H2O
(4) Action on organic compounds
Nitric acid also reacts with organic compounds.
For example: sucrose (cane sugar) is oxidised to oxalic acid by nitric acid.
C12H22 O11 + 36 HNO3 –> 6 (COOH)2 + 36NO2 + 23 H2O
In the presence of sulphuric acid, nitric acid reacts with aromatic compounds forming nitro compounds. This process is called nitration.
For example: it reacts with benzene to form nitrobenzene.
C6H6 + HNO3 —-> C6H5NO2 + H2O
Similarly, phenol reacts with nitric acid in the presence of H2SO4 to give trinitrophenol (known as picric acid).
Nitric acid attacks proteins giving a yellow nitro compound known as xantho protein. Therefore, nitric acid stains skin and renders wool yellow.
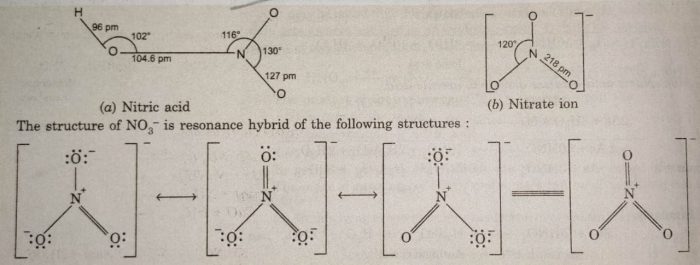
Structure
Gaseous nitric acid has planar structure. Nitrate ion, NO3¯ has also planar symmetrical structure
Brown Ring test for NO3¯ ion
Nitrates give brown ring test with Fe2+ ions in the presence of conc. H2SO4.This is based upon the tendency of Fe2+ to reduce nitrates to nitric oxide which reacts with Fe2+ to form a brown coloured complex.
The test is usually performed by adding dilute FeSO4 solution to an aqueous solution containing NO3¯ ion and then adding conc. H2SO4 slowly along the sides of the test tube. A brown ring at the interface between the solution and sulphuric acid indicates the presence of NO3¯ ion.
3Fe2+ +NO3¯ + 4 H+ —-> NO + 3Fe3+ + 2H2O
Fe3+ + NO + 5 H2O —-> [Fe(H2O)5 NO]2+
Pentaaquanitrosyl iron (II) ion
Uses of Nitric Acid
(i) It is used in the manufacture of ammonium nitrate for fertilizers.
(ii) It is used in the manufacture of sulphuric acid by lead chamber process.
(iii) It is used in the manufacture of explosives such as trinitro toluene (TNT), nitroglycerine, picric acid, etc.
(iv) It is used in the manufacture of dyes, perfumes and silk.
(v) It is used for the manufacture of nitrates for use in explosive and pyrotechnics.
(vi) It is used in picking of stainless steel and etching of metals.
(vii) It is also used as an oxidiser in rocket fuels.
(viii) It is used in the purification of gold and silver as aqua regia.
Leave a Reply