He examined the relationship between atomic weights of the elements and their physical and chemical properties. Among chemical properties, Mendeleev mainly concentrated on the compounds formed by the elements with hydrogen and oxygen because they are highly reactive and hence formed compounds with almost all the elements. The formulae of the hydrides and oxides formed by the … [Read more...] about Mendeleev’s Periodic Law And Table
Class 11
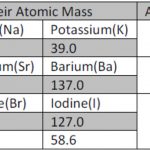
Historical Development of Periodic Table
Periodic table may be defined as the table which classified all the known elements in accordance with their properties in such a way that elements with similar properties are grouped together in the same vertical column and dissimilar elements are separated from one another. Doebereiner's Triad The first attempt towards the classification of elements was made by … [Read more...] about Historical Development of Periodic Table
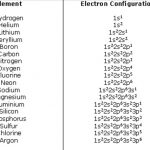
Electronic Configuration of Elements
The distribution of electrons into different shells, sub shells and orbitals of an atom is called its electronic configuration. The electronic configuration of any orbital can be represented as: nlx n is the number of principal shell, l = symbol of the sub shell or orbital, x= number of electrons present in the orbital 4p1 means that p- sub shell of the 4th main shell … [Read more...] about Electronic Configuration of Elements
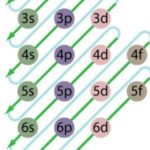
Energy Level Diagram
Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number ( n ) as well as azimuthal quantum number(l). Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important … [Read more...] about Energy Level Diagram
Shapes of Atomic Orbital
An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum. The probability at any point around the nucleus is calculated using schrodinger wave equation and is represented by the density of the points. Shape of s orbital For the coordinates( x, y, z) of the electron with respect to the nucleus, … [Read more...] about Shapes of Atomic Orbital