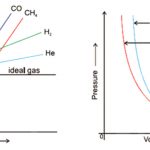
A gas which obeys the ideal gas equation, PV = nRT under all conditions of temperature and pressure is called an ideal gas. There is no gas which obeys the ideal gas equation under all conditions of temperature and pressure. The gases are found to obey the gas laws if the pressure is low or the temperature is high. Such gases are known as real gases. It is found that … [Read more...] about Real Gases
States of Matter
Viscosity
Some liquids like water, ether flow rapidly why some other liquids like glycerine, castor oil flow quite slowly. This internal resistance to flow possessed by a liquid is called its viscosity The liquids which flow slowly, have high internal resistance which is due to strong intermolecular forces and therefore, are said to be more viscous or are said to have high … [Read more...] about Viscosity
Surface Tension
Surface tension is a property of liquid which arises due to the fact that the molecules of the liquid at the surface are in different situation than those in the interior of the liquid. A molecule lying inside the liquid is surrounded by other molecules and so is attracted equally in all directions. Hence ,the net force of attraction acting on the molecule is zero. A … [Read more...] about Surface Tension
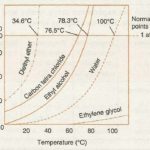
Vapour Pressure
In a liquid, the molecules are quite close together so that there are considerable forces of attraction between them and hence they are held together in a definite volume. The liquids possess fluidity like gases but incompressibility like solid. Properties of Liquid (1) Liquids have no definite shape: They take up the shape of the vessel in which they are put. This is … [Read more...] about Vapour Pressure
Deduction of Gas Laws From kinetic Theory
Boyle's law At constant temperature, the average kinetic energy and hence the average speed of the molecules is constant. The number of molecules present in a given mass of a gas is also constant. Let the volume of a given mass of a gas be reduced to one half of its original volume. The same number of molecules with their same average speed will now have half the original … [Read more...] about Deduction of Gas Laws From kinetic Theory