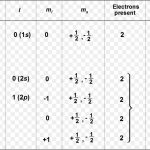
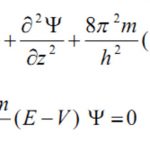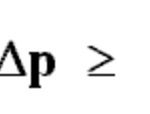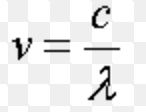Wolfgang Pauli a German physicist in 1925 put forward a principle known after his name as Pauli exclusion principle. No two electrons in an atom can have the same set of four quantum number. In an atom, any two electrons may have the same values for any of the three quantum numbers but the 4th must be different. Any particular orbital is described by three quantum … [Read more...] about Pauli Exclusion Principle
Chemistry
Quantum Numbers
Quantum Numbers An atom contains a large number of orbitals. These are distinguished from each other on the basis of their shape, size and orientation in space. These characteristics of an orbital are expressed in terms of three numbers, called principal, azimuthal and magnetic quantum number. Quantum numbers may be defined as a set of 4 numbers with the help of which we … [Read more...] about Quantum Numbers
Quantum Mechanical Model of an Atom
Quantum mechanics, as developed by Erwin Schrodinger in 1926, is based on the wave motion associated with the particles. For the wave motion of the electron in the three dimensional space around the nucleus, he put forward an equation known as Schrondinger wave equation. where ψ is the amplitude of the wave where the coordinates of the electrons are ( x,y,z) ,E is the … [Read more...] about Quantum Mechanical Model of an Atom
Heisenberg’s Uncertainty Principle
Werner Heisenberg, a German physicist, in 1927 gave a principle about the uncertainty in simultaneous measurement of position and momentum of small particles. Heisenberg's uncertainty Principle states that: It is impossible to measure simultaneously the position and momentum of a small particle with absolute accuracy or certainty. The product of the uncertainty in the … [Read more...] about Heisenberg’s Uncertainty Principle
Dual Nature of Matter and Radiation
Einstein in 1905 suggested that light has dual nature, i.e. wave nature as well as particle nature. Matter wave or a de Broglie wave Louis de Broglie, a French physicist, in 1924, suggested that all microscopic as well as macroscopic objects possesses dual character. The wave associated with the particle is called a matter wave or a de Broglie wave. The wavelength of the … [Read more...] about Dual Nature of Matter and Radiation