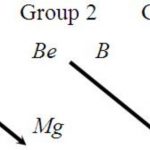
1) Elements in which the last electron enters the s orbital are called s-block elements. Since s-subshell has only one orbital which can accommodate only two electrons, therefore there are only two groups of s-block elements. 2) Elements of group 1 (alkali metals) and group 2 (alkaline earth metals) constitute s- block elements. The elements of these groups contain one or … [Read more...] about s-block elements
Chemistry
Hydrogen Economy
Hydrogen Economy One proposed way to meet the need for new energy sources is to burn hydrogen as a fuel in industry and power plants and possibly also in homes and motor. This proposal is referred to hydrogen economy. Advantages of hydrogen economy 1) Dihydrogen releases large quantities of heat on combustion. The energy released by combustion of fuels like dihydrogen, … [Read more...] about Hydrogen Economy
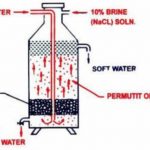
Hard and Soft water
Depending upon its behaviour towards soap solution w.r.t. lather formation, water may be classified as : soft water and hard water. Soft water: Water that produces lather with soap readily is called soft water. For ex: Rain water, distilled water, demineralised water. Hard water: Water which does not produce lather with soap readily is called hard water. For ex: … [Read more...] about Hard and Soft water
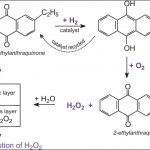
Hydrogen Peroxide
Hydrogen peroxide was discovered by the French Chemist J.L. Thenard in 1818. Preparation 1)From sodium peroxide (Merck's method) Calculated amount of sodium peroxide is gradually added to an . ice-cold solution of 20% H2SO4. Na2O2 + H2SO4 --------> Na2SO4 +H2O2 Upon cooling crystals of Na2SO4·10 H2O separate out and the resulting solution contain about 30% … [Read more...] about Hydrogen Peroxide
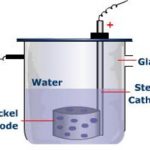
Heavy Water
Heavy Water Heavy water is deuterium oxide (D2O) Preparation 1) By prolonged electrolysis When ordinary water is electrolysed, protium is liberated much more readily than deuterium because of the following reason: a) Being smaller in size, H+ ions have greater mobility as compared to D+ ions. b) Because of lower discharge potential, H+ ions are discharged at … [Read more...] about Heavy Water