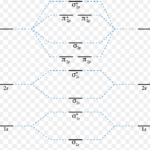
Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ∗(1s) < σ(2s) <σ∗(2s) < π(2px) = π(2py) < σ(2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding … [Read more...] about Energy level diagram for Molecular orbitals
Chemical Bonding and Molecular Structure
Types of Molecular Orbital Formed
Types of molecular orbitals formed 1)If two atomic orbitals overlap along the internuclear axis ,the molecular orbital formed is called σ molecular orbital. 2)If two atomic orbitals overlap sideways, the molecular orbital formed is called π molecular orbital. 3)s orbitals are spherically symmetrical ,their wave function has the same size in all the directions. 4)In … [Read more...] about Types of Molecular Orbital Formed
Shapes Of Molecules
Shape of beryllium fluoride( BeF2) molecule Atomic number of Be = 4 Electronic configuration in ground state is 1s2 2s2 Electronic configuration in excited state is 1s2 2s1 2px1 one 2s orbital and one 2p orbital undergo sp hybridisation to form two half filled sp hybrid orbitals which are oriented at an angle of 180°. They overlap with the half filled orbitals of … [Read more...] about Shapes Of Molecules
Hydrogen Bonding
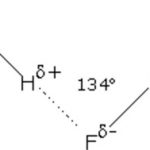
Whenever a molecule contains a hydrogen atom linked to a highly electronegative atom, this atom attracts the shared pair of electrons more and so this end of the molecules becomes slightly negative while the other end become slightly positive. The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This … [Read more...] about Hydrogen Bonding
Molecular Orbital Theory
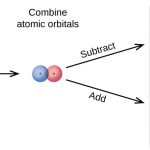
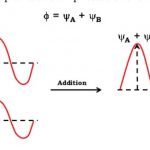
Molecular orbital theory Features of Molecular orbital theory 1) The atomic orbitals overlap to form new orbitals called molecular orbitals. When two atomic orbitals overlap or combine, they lose their identity and form new orbitals. The new orbitals thus formed are called molecular orbitals. 2) Molecular orbitals are the energy states of a molecule in which the … [Read more...] about Molecular Orbital Theory