Types of molecular orbitals formed
1)If two atomic orbitals overlap along the internuclear axis ,the molecular orbital formed is called σ molecular orbital.
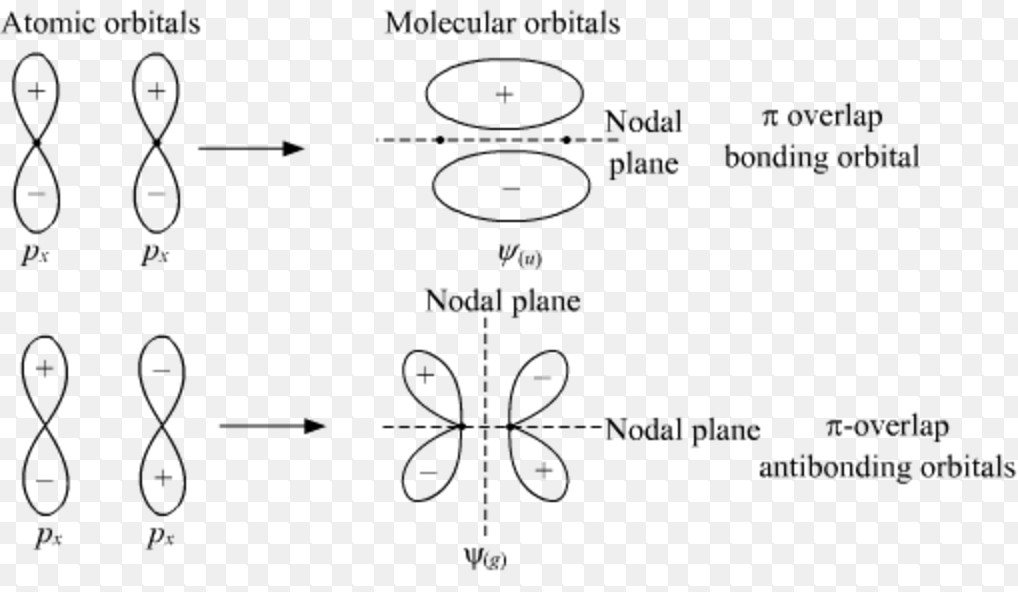
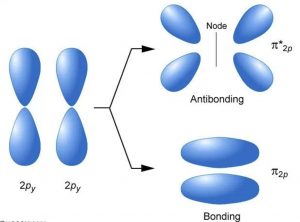
2)If two atomic orbitals overlap sideways, the molecular orbital formed is called π molecular orbital.
3)s orbitals are spherically symmetrical ,their wave function has the same size in all the directions.
4)In p- orbital, one lobe is given a + sign and the other a – sign.
5)Overlapping of + part of the electron cloud of one atom with + part of the electron cloud of second atom implies addition of the atomic orbitals leading to the formation of bonding molecular orbitals.
6)The overlap of + part of the electron cloud of one atom with – part of the electron cloud of the second atom means the subtraction of the atomic orbitals leading to the formation of antibonding molecular orbital.
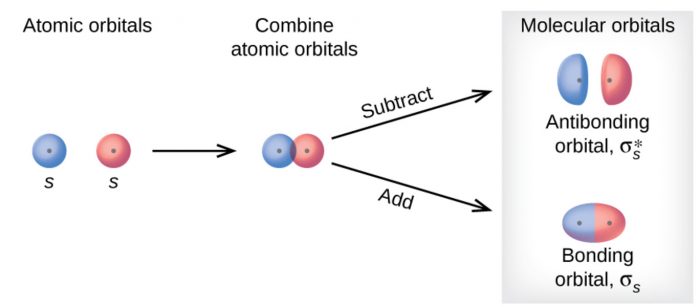
1s with 1s
The wave function of two 1s atomic orbitals can combine in two different ways :
a) When both have the same sign.
b)When they have different signs.
If one of the wave function is assigned a +ve sign, the other may be either +ve or -ve sign. The bonding molecular orbital formed is designated as σ (1s), σ indicating that the overlap is along the internuclear axis and 1s indicating that 1s atomic orbitals have combined to form the molecular orbital. The corresponding antibonding molecular orbital is designated as σ∗
2s with 2s
The bonding and antibonding molecular orbitals formed are designated as σ (2s) and σ∗(2s).
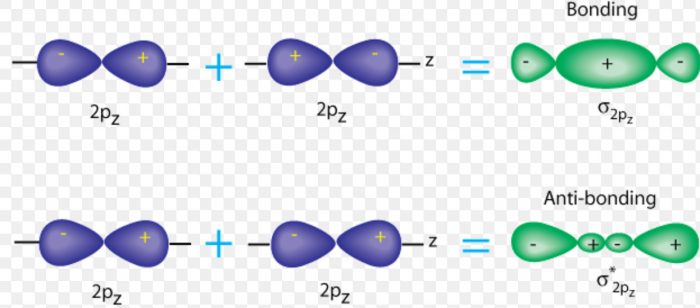
2pz with 2pz
Taking Z-axis as the internuclear axis, the molecular orbitals formed are:
2px with 2px
2py with 2py
Leave a Reply