Surface tension is a property of liquid which arises due to the fact that the molecules of the liquid at the surface are in different situation than those in the interior of the liquid.
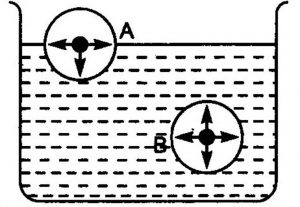
A molecule lying inside the liquid is surrounded by other molecules and so is attracted equally in all directions. Hence ,the net force of attraction acting on the molecule is zero.
A molecule lying at the surface is attracted more by the molecules lying in the bulk of the liquid than by the molecules lying above it in the vapour phase.
A molecule lying at the surface experiences a net inward attraction. As a result of this inward pull on all molecules lying at the surface, the surface behave as if it were under tension.
Surface tension of a liquid is defined as the force acting at right angles to the surface along 1 cm length of the surface.
Thus, the units of surface tension are dynes per centimetre or Newton per metre i.e. Nm-1 in the S.I. system.
As a result of inward pull on the molecules at the surface ,the surface of the liquid tends to the smallest possible area for a given volume of the liquid. This gives the lowest energy state of the liquid.
The drop of a liquid are spherical because for a given volume , a sphere has minimum surface area.
The work in ergs required to be done to increase or extend the surface area by 1 sq cm is called surface energy. The units of surface energy are, therefore , ergs per sq cm or joules per sq m in S.I. system.
Surface energy = work per sq cm= (Force× length) per sq cm
Surface energy = dynes cm-1
Some important results
1)Spherical shape of drops
The lowest energy state of a liquid will be when the surface area is minimum. Surface tension tries to decrease the surface area of the liquid to the minimum. The drops of a liquid are spherical because for a given volume, a sphere has minimum surface area.
2)Fire polishing of glass
Sharp glass edges are heated to make them smooth. This is because on heating ,the glass melts and takes up rounded shape at the edges which has minimum surface area. This is called fire polishing of glass.
3)Rise of a liquid in capillary tube
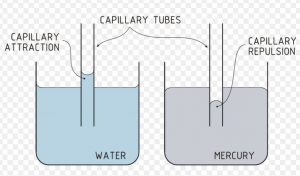
If one end of a capillary tube is put into a liquid that wets glass, it is found that the liquid rises into the capillary tube to a certain height.
This rise is due to inward pull of surface tension acting on the surface which pushes the liquid into the capillary tube. It is because of the same reason that oil rises into the Wick of an oil lamp or water below the surface of the earth rises in the plant or ink in a blotting paper.
In case of liquids which do not wet glass ex Mercury ,the level inside the capillary falls below the level outside, whereas the upper surface of a liquid that wets glass is concave ,that of mercury is convex .
A curved surface of a liquid is known as meniscus.
The attractive forces existing between the molecules of the same substance are known as Cohesive forces. example, between the molecules of water or molecules of Mercury.
The attractive forces existing between the different substances are known as Adhesive forces , eg, between water and glass or Mercury and glass.In case of water taken in a glass tube, adhesive forces are stronger than Cohesive forces whereas it is reverse for Mercury taken in a glass tube.
4)Effect of nature of the liquid on surface tension
Surface tension is a property that arises due to the intermolecular forces of attraction among the molecules of the liquid. Greater are the intermolecular forces of attraction, higher is the surface tension of that liquid.
5)Effect of temperature on surface tension
The surface tension of liquid generally decreases with increase of temperature and becomes zero at the critical temperature. The decrease in surface tension with increase of temperature is due to the fact that with increase of temperature, the kinetic energy of the molecules increases ,and, therefore the intermolecular attraction decreases.
Nice work, thanks for providing notes on Surface Tension for Class 11 Chemistry
Easy and quality notes
Thankyou very much for giving such a great study material………