A given substance can be made to exist in any one of the three states under different conditions of temperature and pressure.
Water which is a liquid under ordinary conditions of temperature and pressure can be converted into steam at 100°C and under 1 atmosphere or into ice by cooling at 0°C
under 1 atmosphere pressure.
Water exist in all the three phases i.e. ice(solid), water (liquid)and water vapour (gas)simultaneously at 273.16 K and 4.58 mm pressure.
Contents
Triple Point
The temperature at which all the three phases of the same substance exist together is called triple point.
The change of state is often accompanied by the absorption or evolution of heat.
Difference between Solid, Liquid and Gas
| Solid | Liquid | Gases |
| (1) They have fixed shape and volume | They do not have fixed shape but have fixed volume | They do not have fixed shape and volume. |
| (2) They cannot be compressed | They cannot be compressed | They can be compressed easily. |
| (3) They have high density | They have moderate density | They have low density. |
| (4) They do not flow | They flow easily | They flow easily. |
| (5) They do not fill their container | They do not fill their container | They fill their container. |
| (6) The forces of attraction are strong | The forces of attraction are less strong than solids | The forces of attraction are weak. |
| (7) Kinetic energy is least | Kinetic energy is more than solids | Kinetic energy is maximum. |
| (8) Particles are closely packed | Particles are not close as in solids | Particles are much farther apart from one another. |
| (9) For Example: Chair, table, chalk, book | For Example: Water, petrol, cold drinks | For Example: Oxygen, nitrogen, helium. |
Two more states of matter have been found to exist :
(1) Plasma state which consists of a mixture of electrons and positively charged ions formed due to superheating of the gaseous state.
Example: in the sun or stars.
(2) Fifth state consists of a supercooled liquid in which the atoms lose their individual identity and condensed to form a single super atom.
Intermolecular Forces
The forces of attraction existing among the molecules of a substance are called intermolecular forces.
Intermolecular forces, i.e. forces which exist within same molecule or a polyatomic ion ,affect the chemical properties of the substance.
Greater the intermolecular forces, higher is the boiling point.
The intermolecular forces arises due to following interactions:
(1) Dipole- dipole interaction
Polar molecules have permanent dipole. These forces of attraction occur among the polar molecules.
The positive pole of the one molecule is thus attracted by the negative pole of the other molecule.
For Example: In HCl, chlorine being more electronegative acquires a slight negative charge whereas the hydrogen end becomes slightly positively charged. The dipole dipole interactions then take place among the HCl molecules as:
The magnitude of dipole-dipole forces in different polar molecules can be predicted on the basis of polarity of the molecules, which in turn depends upon the electronegativities of the atoms present in the molecule and the geometry of the molecule.
The existence of these forces was studied by keesom in 1912.Hence ,these forces are also called Keesom forces and the effect is called orientation effects.
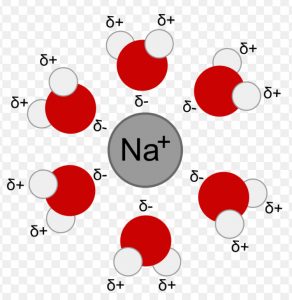
(2) Ion dipole interactions
This is the attraction between an ion and a polar molecule. When NaCl is dissolved in water, the polar water molecules are attracted towards Na+ as well as towards Cl− ion.The strength of this interaction depends upon the charge and size of the ion and the magnitude of dipole moment and size of the polar molecule.
Due to greater charge density on the cation, this interaction is usually stronger with the cation than with the anion having the same charge but bigger size.
CCl4 being non-polar ,cannot interact with Na+ and Cl− ions.Hence NaCl is insoluble in CCl4.
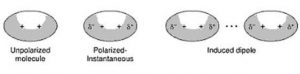
(3) Ion – induced dipole interaction
A non-polar molecule may be polarised by the presence of an ion near it, i.e. it becomes an induced dipole. The interaction between them are called ion induced dipole interaction.The strength of these interactions depend upon the charge on the ion and the ease with which the non polar molecules get polarized. A cation polarizes the molecule by attraction of the electron cloud whereas an anion does it by repulsion.
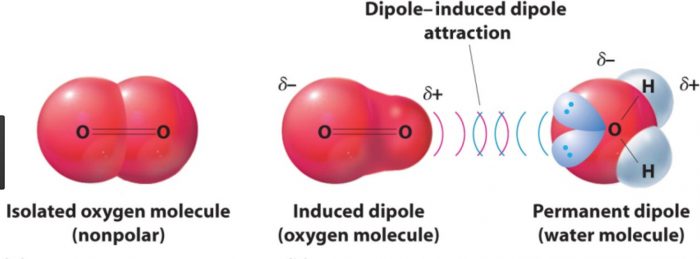
(4) Dipole- induced dipole interaction
A non-polar molecule may be polarized by the presence of a polar molecule near it, thereby making it an induced dipole. The interactions between them are then called dipole induced dipole interactions.
Strength will depend upon the strength of the dipole and the ease of polarizability of the non polar molecules.
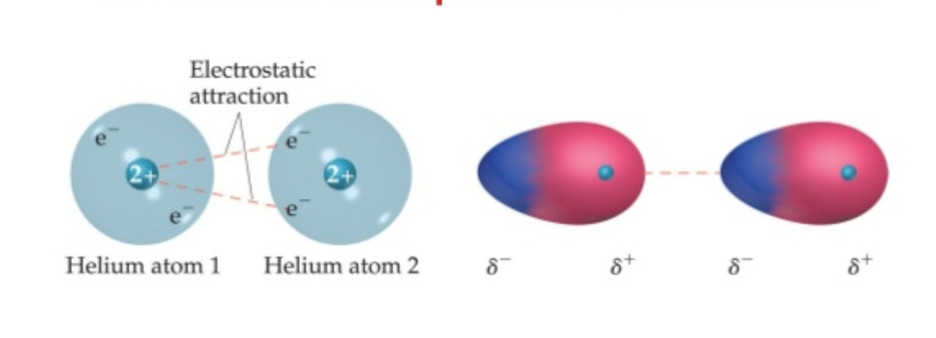
(5) London forces or dispersion London
These forces were proposed by Fritz London in 1930. Hence they are termed as London forces. These forces arise from the motion of the electron. It is believed that at any instant of time, the electron cloud of the molecule may be distorted so that an instantaneous dipole used is produced in which one part of the molecule is slightly more negative than the rest.
The instantaneous dipole induce dipole in the neighbouring molecule. These are then attracted to each other exactly in the same way as the permanent dipole. The forces of attraction between the induced dipole are called London dispersion forces.
London forces are the weakest intermolecular forces. Their magnitude depends upon the following factors:
(1) Size of the molecule: Larger or more complex are the molecule, greater is the magnitude of London forces. This is due to the fact that the large electron cloud are easily distorted or polarised. Since the larger molecular size amounts to larger molecular mass, it is suggested that the magnitude of London forces increases with increasing molecular mass.
For noble gases ,as we move down the group, the size of the atom increases ,i.e. size of the electron cloud increases which gets more and more distorted. As a result, London forces increase and so do their boiling point.
(2) Geometry of the molecule: n-pentane and neo- pentane have the same molecular formula yet the boiling point of n-pentane is about 27° higher than that of neo-pentane. The difference is due to the different shapes of the two molecule: n-pentane being a zigzag chain where as neo-pentane is nearly spherical.
The overall attraction between molecule is greater in the case of n- pentane because there are more sites of interaction, the molecules are able to come in contact with the entire length of the molecule. In case of neo- pentane there is less contact and hence less force of attraction.
Intermolecular Forces vs Thermal Energy
(1) Intermolecular forces are the forces of interaction between the molecules of that substance which try to bring the molecule closer.
(2)Thermal energy possessed by the molecules due to temperature which result into the movement of the molecule and hence tries to keep them apart.
In gases, the intermolecular forces of attraction are weakest while thermal energy is highest. In solid ,intermolecular forces of attraction are strongest while thermal energy is minimum.
(1) A solid has rigidity because thermal motion is too weak to overpower the strong intermolecular forces of attraction. In a gas, thermal energy is so high that the molecules cannot come close together. Hence there are large empty spaces between them .In liquids there is a balance between the attractive intermolecular forces and thermal energy. Hence, molecule in a liquid exist together, i.e. it is a condensed state of matter but there is no rigidity.
(2) A solid melts on heating because on heating, the thermal motion increases.
(3) In both liquid and solid, their molecules exist together ,i.e. both of them are condensed States of matter having sufficiently strong intermolecular forces of attraction. Hence they exhibit very low compressibility. In gases, there are large empty spaces between the molecules therefore they possess high compressibility.
Melting point is the temperature at which a solid melts to form a liquid.
Boiling point is the temperature at which a liquid changes into vapour.
The melting or the boiling point of a substance depends upon the magnitude of the forces existing among its constituent particles. The greater is the magnitude of these forces, higher is the melting point or the boiling point.
Ionic Compound
In ionic compounds, the constituent particles are positively and negatively charged ion. The forces of attraction between the oppositely charged ion being electrostatic in nature, are the strongest. Therefore, ionic compounds have very high melting and boiling point.
Covalent Compound
In covalent compound, the force of attraction among the molecules are weak van der waals forces. They have low melting and boiling point.
Polar molecules such as HCl have greater intermolecular forces of attraction than non polar molecules such as H2, O2, He, Ne, CH4, which have only weak london forces.
Polar molecules such as HF, NH3, H2O which can form hydrogen bonds have higher boiling point than molecules which do not form hydrogen bonds.

nice explanation
Thanks for sharing your experience