Contents
Matter
Matter is defined as anything that occupies space and possesses mass.
Classification of Matter
(1) Physical classification
(a) Solid
(b) Liquid
(c) Gases
| Solid | Liquid | Gases |
| (1) They have fixed shape and volume | They do not have fixed shape but have fixed volume | They do not have fixed shape and volume |
| (2) They cannot be compressed | They cannot be compressed | They can be compressed easily |
| (3) They have high density | They have moderate density | They have low density |
| (4) They do not flow | They flow easily | They flow easily |
| (5) They do not fill their container | They do not fill their container | They fill their container |
| (6) The forces of attraction are strong | The forces of attraction are less strong than solids | The forces of attraction are weak. |
| (7) Kinetic energy is least | Kinetic energy is more than solids | Kinetic energy is maximum. |
| (8) Particles are closely packed | Particles are not close as in solids | Particles are much farther apart from one another. |
| (9) For Example : Chair, table, chalk, book | For Ex: Water, petrol, cold drinks | For Ex: Oxygen, nitrogen, helium |
Vapours represent a gaseous state of a substance which is liquid at room temperature.
A substance which is in gaseous state at room temperature is called a gas.
For Ex: Ammonia is a gas but on heating water forms vapours.
(2) Chemical classification
All kinds of matter are classified into two types:
(a) Homogeneous
(b) Heterogeneous
Material is said to be homogeneous if it has uniform composition and identical properties throughout Or a material is said to be homogeneous if it consist of only one phase.
A material is said to be heterogeneous if it consists of a number of phases. The different phases are separated from each other by distinct boundaries.
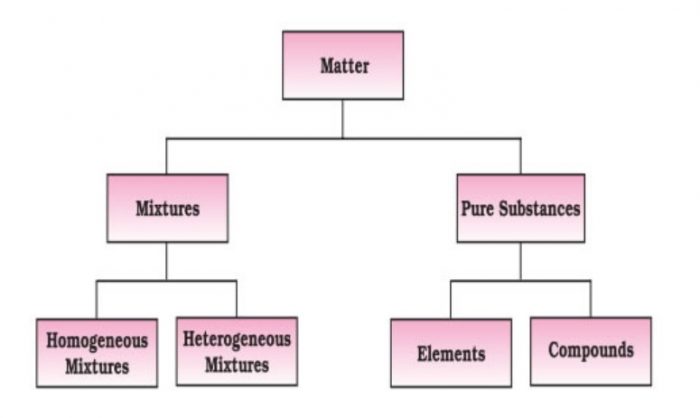
Pure Substance
A pure substance is one which is made up of only one kind of particles (atoms or molecules).
Pure substance are of two types:
(1) Elements
(2) Compounds
Mixture
A mixture is one which contains two or more different kinds of particles(atoms or molecules).
Mixtures are of two types:
(1) Homogenous mixture
(2) Heterogeneous mixture
Elements
An element is usually defined as the simplest form of a pure substance with definite physical and chemical properties and which can neither be broken into nor built from simpler substances by any chemical or physical method.
An element contains only one kind of particles. These particles may be atoms or molecules.
For example: carbon, sulphur, iron, gold, hydrogen, oxygen, nitrogen, silver etc.
The number of elements known to date is 114 .out of these 92 occur in nature in the earth crust and the remaining have been prepared artificially in the laboratory through reactions.
Types of Elements
(1) Metals
(2) Non metals
(3) Metalloids
| Metals | Non-metal |
| (1) They are malleable | They are not malleable |
| (2) They are ductile | They are not ductile. |
| (3) They are good conductors of heat and electricity. | They are bad conductors of heat and electricity. |
| (4) They are lustrous | They are not lustrous. |
| (5) They have high tensile strength | They have low tensile strength |
| (6) They are sonorous | They are not sonorous |
| (7) They are hard | They are soft |
Metalloids
The elements whose properties are intermediate between those of metals and non-metals are known as metalloids.
For Example : Silicon, Germanium, Arsenic, Tellurium.
Compounds
Compounds are pure substances containing more than one kind of element or atom.
In compounds the two elements are in a fixed proportion by mass and which can be decomposed into its constituent elements by suitable chemical methods. The properties of a compound are completely different from those of its constituent elements.
For example : Water is a compound containing hydrogen and oxygen combined together in a fixed proportion of 1:8 by weight.
It can be decomposed into its constituent elements hydrogen and oxygen by passing electricity through water. Water are completely different from its constituent hydrogen and oxygen.
For Example : Carbon dioxide, Sulphur Dioxide, hydrochloric acid, nitric acid, washing soda, common salt are examples of compounds.
Types of compounds
All the compounds may be divided into two categories :
(1) Organic compounds
Organic Compounds are the compounds containing carbon and few other elements like carbon, Hydrogen, oxygen, nitrogen, sulphur, halogens. They were originally obtained only from plants and animals.
(2) Inorganic compounds
Inorganic Compounds are the compounds containing any two or more elements out of more than one 114 elements known.
Mixtures
A material containing two or more substances in any proportion is called a mixture. The properties of a mixture are the properties of its constituents. A mixture can be separated into its constituent by simple physical method.
Types of mixture
(1) Homogeneous
(2) Heterogeneous
| Homogeneous Mixture | Heterogeneous Mixture |
| (1) Those mixtures in which the substances are completely mixed together and are indistinguishable from one another. | Those mixtures in which the substances remain separate and one substance is spread throughout the other. |
| (2) They have uniform composition throughout its mass. | They do not have uniform composition. |
| (3) It has no visible boundaries of separation between various constituents. | It has visible boundaries. |
| (4) For Ex: Sugar solution, Salt solution, Alcohol and water, Soft drinks etc. | For Ex: Sugar and sand, Salt and Sand, Milk, Soil, Blood, Starch, Muddy water etc. |
Difference between Mixture and Compound
| Mixture | Compounds |
| (1) A mixture can be separated into its constituents by the physical processes. | A compound can not be separated into its constituents by the physical processes. |
| (2) A mixture shows the properties of its constituents. | A compound does not shows the properties of its constituents. |
| (3) Energy is usually neither given out nor absorbed in the preparation of a mixture. | Energy is usually given out or absorbed during the preparation of a compound. |
| (4)The composition of a mixture is variable, the constituents can be present in any proportion by mass. | The composition of a compound is fixed, the constituents are present in fixed proportion by mass. |
| (5) A mixture does not have a fixed melting and boiling point. | A compound has a fixed melting and boiling point. |
Atom
An atom is the smallest particle of an element which may or may not be capable of independent existence.
For example : Atoms of iron, copper, Silver, Gold can exist freely whereas atoms of hydrogen, oxygen, nitrogen cannot exist freely.
Molecule
A molecule is the smallest particle of an element or a compound which can exist freely.
Molecules may be classified into two categories:
(1) Molecules of elements: They are made up of only one kind of atom, they are called homoatomic or homonuclear molecules.
The atomicity of metal elements like sodium, magnesium, aluminum, copper, iron etc. is taken as 1. They are called as Monoatomic molecules.
(2) Hydrogen, Nitrogen, Oxygen, Chlorine, bromine etc. have two atoms in their molecules. So the atomicity is 2.They are called as diatomic molecules.
(3) Ozone has 3 atoms in its molecules, so the atomicity is 3.They are called as triatomic molecules.
(4) Phosphorus has 4 atoms in its molecules, so the atomicity is 4.they are called as tetra-atomic molecules.
Molecules of compounds :They are made up of atoms of different elements and hence are called heteroatomic or heteronuclear molecules.
They may be diatomic, triatomic depending upon the number of atoms present in a molecule of the compound .
For Example: HCl, water, carbon dioxide, ammonia, methane etc.
Thanks for supporting us by providing notes on Classification of matter for class 11
Allah give you success in your aims
THANK YOU SO MUCH FOR ACCESSIBILITY OF THESE NOTES .THIS APP IS LIFE GIVING FOR TEACHERS .WHO ARE TAKING ONLINE CLASSES IN THE OUTBREAK OF CORONA ACROSS THE WORLD
Ok, thanks for you.
you are right this is better for students like us.because we are taught online by the help of these sites or the teachers who are trying to tech us.
hey you are great you are like that godess
thanks for our helping
Thanks u so much
Thank you