Einstein in 1905 suggested that light has dual nature, i.e. wave nature as well as particle nature.
Contents
Matter wave or a de Broglie wave
Louis de Broglie, a French physicist, in 1924, suggested that all microscopic as well as macroscopic objects possesses dual character. The wave associated with the particle is called a matter wave or a de Broglie wave.
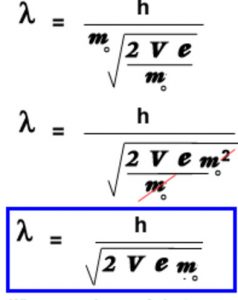
The wavelength of the wave associated with any material particle was calculated as:
In case of photon, if it is assumed to have a wave character, its energy is given by
E = hν
where ν is the frequency of the wave and h is Planck’s Constant.
If the photon is supposed to have particle character, its energy is given by
E = mc2
where m is the mass of the photon and c is the velocity of light.
The above equation is called de Broglie Equation and λ is called de Broglie wavelength.
de Broglie wavelength of a particle is inversely proportional to its momentum.
Significance
De Broglie equation relate the particle character with the wave character of matter.
Consider a ball of mass 0.1 kg moving with a speed of 60 m/s.
From de Broglie equation:
λ = h/mv
where h= 6.62 × 10-34
m= 0.1 kg
ν= 60 m/s
we λ = 10-34
Wavelength is too small for ordinary observation.
It has significant only in case of microscopic particles. de Broglie relationship has no significance in everyday life. That is why we do not observe any wave nature associated with the motion of a running car or a cricket ball.
Verification of dual character of electrons
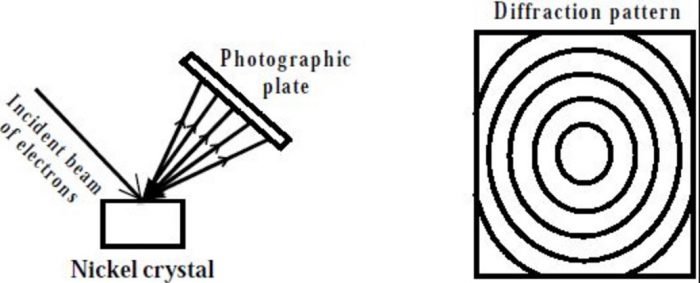
(1) Davisson and Germer’s experiment
Davisson and Germer in 1927 observed that when a beam of electrons is allowed to fall on the surface of nickel crystal and the scattered or reflected rays are received on a photographic plate, a diffraction pattern (consisting of number of concentric rings) similar to that produced by X-ray, is obtained. Since X-ray are electromagnetic waves i.e. they are confirmed to have wave character, therefore electrons must also have wave character.
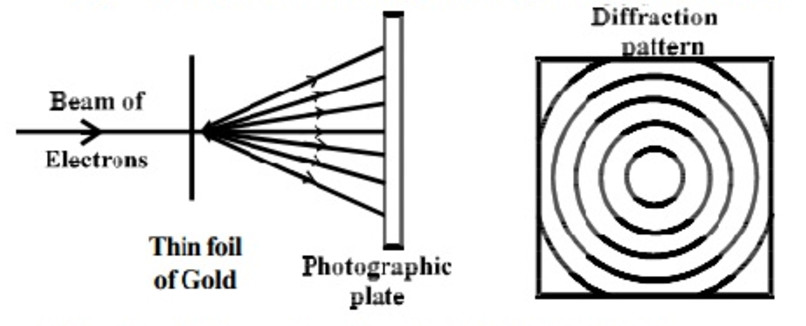
(2) Thomson’s experiment
He performed experiment with thin foil of gold in place of nickel crystal. He observed that if the beam of electrons after passing through the thin foil of gold is received on the photographic plate placed perpendicular to the direction of the beam, a diffraction pattern is observed.
Verification of Particle Character
When an electron strikes a zinc sulphide screen, a spot of light known as scintillation is produced. Since a scintillation is localised on the zinc sulphide screen, therefore, the striking electron which produces it also must be localised and not spread out on the screen. The localised character is possessed by the particles .Hence electrons has particle character.
(2) Milliken Oil Drop experiment for determination of charge on electron also show that electron has particle character.
(3) The phenomenon of black body radiation and Photoelectric effect also Prove the particle nature of radiation.
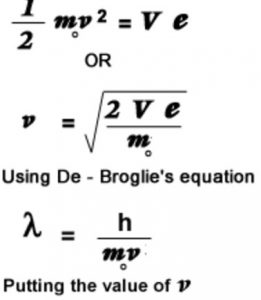
De Broglie wavelength of the electron from the potential applied to accelerate it
Derivation of Bohr’s postulate of angular momentum from de Broglie equation
According to Bohr’s model, the electron revolves around the nucleus in circular orbits.
According to de Broglie concept, the electron is not only a particle but has a wave character.
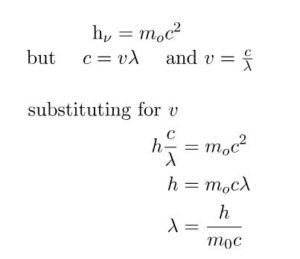
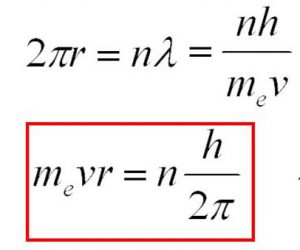
Thanks
Nice notes about wave.
very very helpful …………….
thnx a lot respected mam
good and excellent notes ……..
easy to study and learn……………
best notes for me thank you mam for such notes
Thanks mam,
For these notes about wave
They are very helpful