William crookes in 1879 studied the conduction of electricity through gases at low pressure.
EXPERIMENTAL SET UP
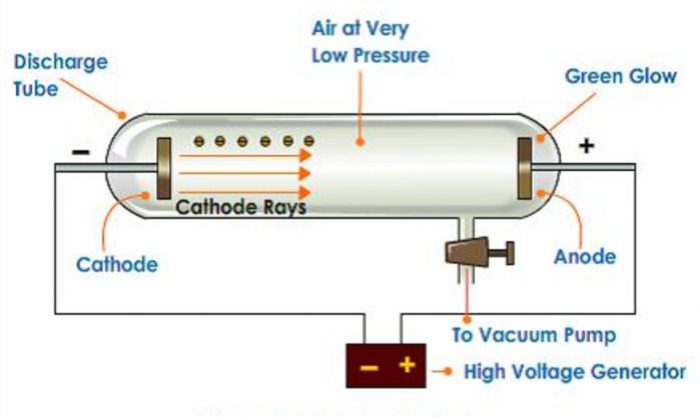
1) He took a discharge tube which is a long glass tube, sealed at both the ends and fitted with two metal electrodes.
2) It has a side tube fitted with a stop cock which can be connected to a vacuum pump to reduce the pressure of the gas inside to any desired value.
When high voltage of about 10000 volts is applied between the electrodes the following results are observed at different pressures :
1) When the gas pressure inside is one atmosphere, no current flows between the electrodes.
2) When the pressure is reduced to about 10 millimetre, the current starts flowing between the electrodes and coloured glow is observed, the colour depending upon the nature of the gas taken.
3) When the pressure is for the reduced to about 0.01 millimetre, the glow between the electrodes disappear but the current continuous to flow and if a perforated anode is used, a faint greenish glow is observed on the glass walls behind the anode. This shows that some invisible rays are emitted from the cathode which passed through the holes of the anode and strike the wall. These rays were called as cathode rays.
Properties of cathode rays
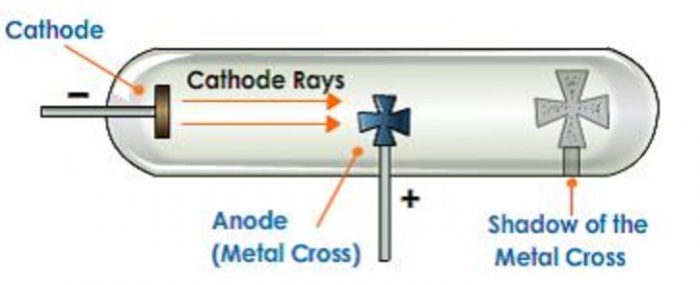
1)They produce a sharp Shadow of the solid object placed in their path. This shows that cathode rays travel in straight line.
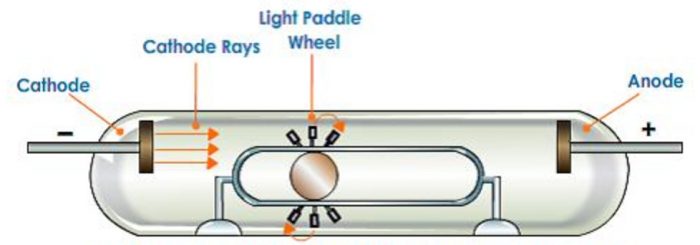
2)If a light paddle wheel mounted on an axle is placed in their path, the wheel begins to rotate. This shows that cathode rays are made up of material particles.
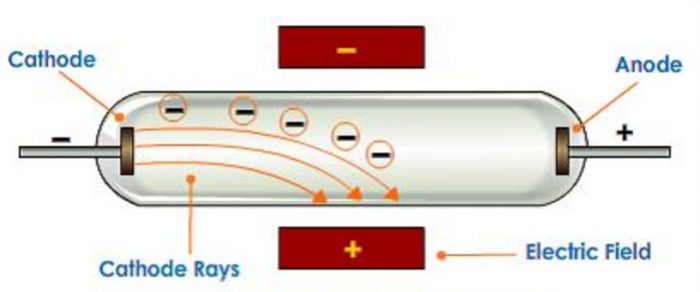
3)When an electric field is applied on the cathode rays, they are deflected towards the positive plate of the electric field. This shows that cathode rays carry negative charge.
4)When cathode rays strike a metal foil, the latter becomes hot. This indicates that cathode ray produce heating effect.
5)They produce green fluorescence on the glass walls of the discharge tube as well as on certain other fluorescent substances such as zinc sulphide.
6)They possess penetrating effect ie they can easily pass through thin foils of metals.
The negatively charged material particles constituting the cathode rays are called electrons.
For determination of the ratio of charge/mass of electrons
The experiment were carried out by J.J. Thomson in 1897. He used different discharge tubes fitted with electrodes of different metals. He placed different gases in the tube. He found every time that the ratio of charge/mass of the electron was the same.
The value was found to be 1.76 × 108 coulombs/ g
For the determination of the charge on the electron
The charge on the electron was found by R.A. Millikan in 1917 with the help of his Oil Drop experiment.
The value was found to be 9.11 × 10-10 g
An electron is that fundamental particles which carries 1 unit negative charge and has a mass nearly equal to 1/1837 th of that hydrogen atom.
Hmm it appears like your website ate my first comment (it was extremely long) so I guess I’ll just sum it up what I
submitted and say, I’m thoroughly enjoying your blog. I as well am an aspiring
blog writer but I’m still new to the whole thing.
Do you have any tips and hints for inexperienced blog
writers? I’d genuinely appreciate it.
Nice article. Easily understandable for students.
Mrs. Shilpi I really appreciate your initiative starting this blog with a mindset for free education to all.
It’s soo usefull to me,Thankyou .
Good studymeterial for the student who are first in 11th or 12th
Its very useful to every student.
I hope those student who read carefully it ,they must go first rank.
amazing experience the quality of explaining it was mesmerising so it is totally beautiful experience and i am alway willing to study like this questions and this is the best approach to the students .than you so much
I liked the material. It was easy to understand. Thank you. I appreciate your intent of free knowledge to all.
It’s so useful because the book is really confusing. So I just prefer short notes. Thank you ☺️
This is so easy and help full for me with the help of this is easily complete my assignment