Hybridisation is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new orbitals of equal energies and identical shape.
The new orbitals thus formed are known as hybrid orbitals.
Important points about hybridisation
1)Only those orbitals which have approximately equal energies and belong to the same atom or ion can undergo hybridisation.
2)Number of hybrid orbitals produced is equal to the number of atomic orbitals mixed.
3)It is not necessary that all the half filled orbitals must participate in hybridisation. Even completely filled orbitals with slightly different energies can also participate.
4)Hybridisation never take place in isolated atoms but it occurs only at the time of bond formation.
5)One can tell the shape of a molecule by knowing the kind of hybridisation involved.
6)The bigger lobe of the hybrid orbital always have positive sign while the smaller lobe on the opposite side has negative sign.
Types of hybridisation
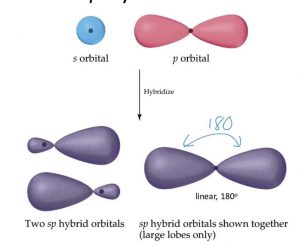
1) sp hybridisation
When one s and one p orbital belonging to the same main shell of an atom mix together to form two new equivalent orbitals ,the type of hybridisation is called sp hybridisation.
The new orbitals formed are called sp hybrid orbitals.
They are collinear with an angle of 180° .
Each of the hybrid orbitals formed has 50% s character and 50% p character.
The remaining two p orbitals which do not participate in hybridisation remains as such.
Example:
1)All compounds of beryllium like BeF2 , BeH2
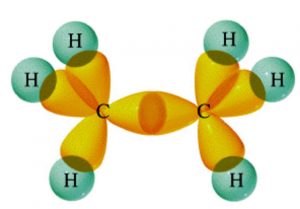
2)All compounds of carbon containing triple Bond like C2H2.
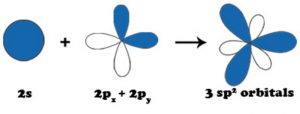
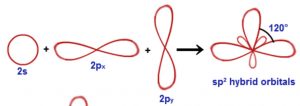
2) sp2 hybridisation
When one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbitals, the type of hybridisation is called sp2 hybridisation.
The new orbitals formed are called sp2 hybrid orbitals.
All the three hybrid orbitals remain in the same plane making an angle of 120° with one another.
Each of the hybrid orbitals formed has 1/3rd s character and 2/3rd p character.
The non-participating p-orbital ,if half filled ,can form bond with other atoms having half filled orbitals.
For example:
1)All compounds of Boron i.e. BF3 , BH3
2)All compounds of carbon containing carbon carbon double bond.
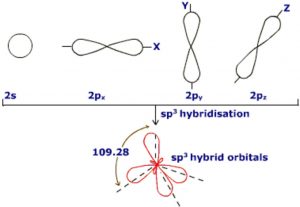
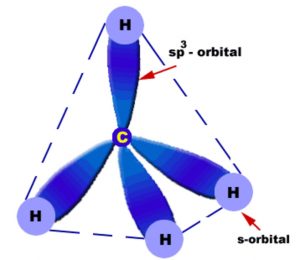
3) sp3 hybridisation
When One s orbital and 3 p orbitals belonging to the same shell of an atom mix together to form four new equivalent orbital ,the type of hybridisation is called a tetrahedral hybridisation or sp3 .
The new orbitals formed are called sp3 hybrid orbitals.
These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28’ with one another.
Each sp3 hybrid orbital has 25% s character and 75% p character.
Example
Methane, ethane
What are the possible questions that can come from this ?
YOUR NOTES ARE VERY GOOD FOR WE THE STUDENTS
very good
Nice notes Thanks
excellent
Superb work mam thank you so much
Thank you mam for this notes
Please keep on providing notes
Thank you mam!
thank you for lightening the blind
YOUR NOTES ARE REALLY HELPFUL FOR US TO UNDERSTAND THE CONCEPTS
Really your article is very helpful. thank you for sharing.
Thanks for explaining in such a simplistic manner.
great for revision purpose
The 3rd point of “Importance points about hybridization”, really helps me a lot…….
And really a nice, pin point and ever sime note…..
Thank you mam…..
Thank you so much ma,am for providing Notes for students