Contents
Solubility of Gases and Solids in Liquids
Solubility of a substance expresses the maximum amount of it which can be dissolved in a specific amount of solvent.
The solubility of a substance depends upon
(i) the nature of solute
(ii) nature of the solvent
(iii) temperature and
(iv) pressure.
Solubility of Solids in Liquids
The solubility of solids in liquids varies greatly with the nature of the solid and liquid, temperature and to a much lesser degree the pressure of the system.
When a solid (solute) is added to the solvent, the solute dissolves because its particles go into the liquid and its concentration in the solution increases. This process is known as dissolution.
Some solute particles in solution collide with the solid solute particles and get precipitated out. This process is called crystallisation.
The process of dissolution continues until the solution attains a certain maximum concentration.
A solution in which no more solute can be dissolved at the same temperature and pressure is called saturated solution.
A solution in which more solute can be dissolved at the same temperature is called unsaturated solution.
At the saturated solution stage, an equilibrium gets established between the process of dissolution and crystallisation. The number of solute particles going into the solution will be equal to the number of solute particles separating out and a state of dynamic equilibrium is reached.
Solute + Solvent ⇔ Solution
In saturated solution, the concentration of solute in the solution will remain constant under the given conditions i.e., temperature and pressure.
The maximum amount of solute that can be dissolved by the solvent at a particular temperature is called its solubility.
The solubility of a substance at a given temperature is defined as the amount of the solid that dissolves in 100 g of the solvent at a given temperature to form a saturated solution.
The solubility is also expressed as molar solubility which gives the molar concentration of a substance in a saturated solution.
For example: If the concentration of glucose in its saturated solution at 20°C is 6 mol L-1 so its molar solubility is reported as 6 mol L-1. Thus, the concentration of the solute has the highest value in saturated solution.
The solubility of a sold in liquid depends upon the following factors:
(1) Nature of solute and the solvent
A solid dissolves in a liquid (solvent) if the intermolecular interactions are similar in solute and the solvent.
(a) Ionic (or polar) compounds dissolve more readily in polar solvents like water and are very little soluble or almost insoluble in non-polar solvents like benzene, ether, carbon tetrachloride.
(b) Non-polar (covalent or organic) compounds are soluble in non-polar solvents like benzene, ether, carbon tetrachloride but are very little soluble in water (polar solvent).
For example: common salt (sodium chloride) and sugar dissolve readily in water.
Naphthalene and anthracene (non-polar compounds) are not soluble in water but dissolve readily in benzene.
(2) Effect of Temperature
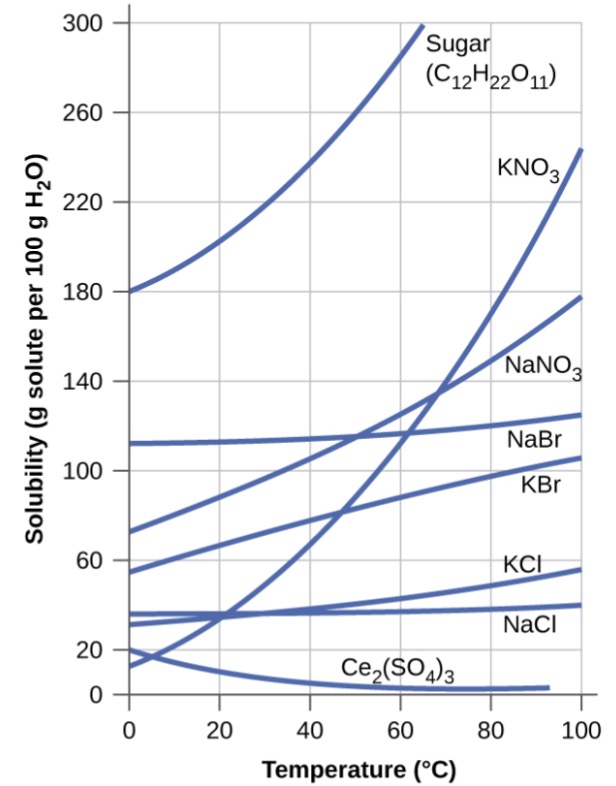
Temperature has a marked effect on the solubility of a solid in a solvent. The solubility may increase or decrease with increase in with temperature. It is observed that most ionic and molecular solids become more soluble at higher temperature.
The variation of solubility of a solid with temperature depends upon the enthalpy of solution.
(a) The solubility of solutes increases with increase in temperature: The solubility of most of substances such as sodium nitrate (NaNO3), ammonium chloride (NH4Cl), potassium iodide (KI) increases with rise in temperature. This is because the dissolution process for these substances is endothermic (ΔsolH > 0).
Solute+ Solvent + Heat ⇔ Solution ΔsolH =+ve
Since these substances absorb heat on forming solution and therefore, their solubility increases with increase in temperature in accordance with Le-Chatelier’s principle.
(b) The solubility of solids decreases with increase in temperature: The solubility of some substances like lithium sulphate (Li2SO4), cerium sulphate (Ce2(SO4)3), sodium carbonate monohydrate (Na2C03·H20) decrease with rise in temperature. This is because their dissolution process is exothermic. ( ΔsolH < 0)
Solute + Solvent ⇔ Solution + Heat ΔsolH =-ve
Since these dissolve with evolution of heat, a decrease of solubility with temperature is expected in accordance with Le-Chatelier’s principle.
(c) The solubility shows irregular behaviour with increase in temperature: For some substance the solubility behaviour is not regular.
For example: The solubility of sodium sulphate (Na2SO4) increase upto a certain temperature and then decreases as the temperature is further raised.
The temperature corresponding to the break in the solubility curve is known as the transition temperature.
Note
(1) If the solute dissolves with absorption of heat (endothermic process), the solubility increases with rise in temperature.
(2) If the solute dissolves with evolution of heat (exothermic process), the solubility decreases with rise in temperature.
(d) Effect of pressure: The effect of pressure on the solubility of solids in liquids is generally very small . This is because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.
Solubility of Gases in Liquids
Gases dissolve in liquids to form homogeneous solutions. The solubility of gas in a liquid depends upon:
(i) The nature of the substance (solute)
(ii) The nature of the solvent
(iii) Temperature of the solution
(iv) Pressure
Gases like nitrogen, hydrogen, oxygen, helium, etc. dissolve in water only to a small extent whereas the gases like ammonia, sulphur dioxide, hydrogen chloride, etc. are highly soluble in water. The most soluble gases are those which chemically react with the liquid solvent
The solubility of a gases in liquids is greatly influenced by pressure and temperature:
(i) Effect of temperature: The solubility of a gas decreases with increase of temperature. This is because gases dissolve in a liquid with the evolution of heat i.e. exothermic process. Therefore, in accordance with Le-Chatelier’s principle, the increase in temperature will result in decrease in the solubility of the gas.
(ii) Effect of pressure: The solubility of gases increases with increase of pressure. This behaviour is also in accordance with Le-Chatelier’s principle.
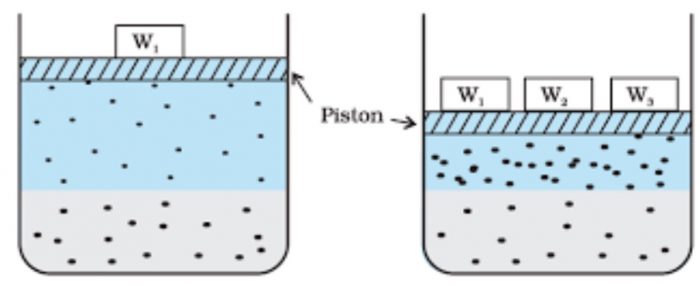
Consider a gas in dynamic equilibrium with a solution.The lower part represents the solution and the upper part is gaseous system at a pressure p and temperature T.
Since there is dynamic equilibrium the number of gas molecules entering the solution is equal to the number of dissolved molecules leaving the solution phase.
Now increase the pressure over the solution phase by compressing the gas to a smaller volume.This will increase the number of gaseous particles per unit volume over the solution.
As a result, the more molecules will be striking the surface of the liquid and hence more molecules will dissolve and the solubility of gas will increase until a new equilibrium is reached. Thus, increasing the pressure of a gas above the solution, increases the solubility of the gas.
The concentration of the dissolved gas is proportional to the pressure on the gas above the solution.
Much Thanks…very useful notes
Great work ,thanks for this excellent job done