Contents
- 1 Electrochemical Cells
- 2 Electrolytic cells or Electrolysis
- 3 Electrochemical Cell or Galvanic Cell
- 4 Functions of the salt bridge
- 5 Functioning of the cell when external opposing potential is applied
- 6 Representation of an Electrochemical Cell
- 7 Memory Aid
- 8 Representation of Some Common Cells by Cell Notation
Electrochemical Cells
The chemical changes which involve the flow of electric current are called electrochemical changes.
These are broadly of two types:
1) Electrochemical cells or Galvanic cells
These constitute the electrochemical reactions in which chemical energy is converted to electrical energy. In these cells, spontaneous redox reaction is used to generate an electric current.
The devices in which chemical energy of a spontaneous redox reaction is converted into electrical energy are called electrochemical cells or galvanic cells. In these devices, the Gibbs energy of the spontaneous redox reaction is converted into electrical work which may be used for running a motor or other electrical gadgets like heater, fan, geyser, etc.
An early example of a galvanic cell is a Daniel cell which was invented by the British chemist John Daniel in 1836. Daniel cell was constructed on the basis of the following spontaneous redox reaction :
Zn (s) + Cu2+ (aq) ⇔ Zn2+ (aq) + Cu (s)
Electrolytic cells or Electrolysis
These constitute the electrochemical reactions in which electrical energy is converted into chemical energy. In these cells electrical energy (electrical current) is used to drive redox reactions which have positive Gibbs energy (ΔG) and are non-spontaneous.
The phenomenon of chemical changes taking place by the passage of electrical energy from an external source is called electrolysis. The devices or cells used to carry out electrolysis are called electrolytic cells.
For example: When electric current is passed through molten sodium chloride, sodium is produced at cathode and chlorine is liberated at anode.
2NaCl ——–> 2Na + Cl2
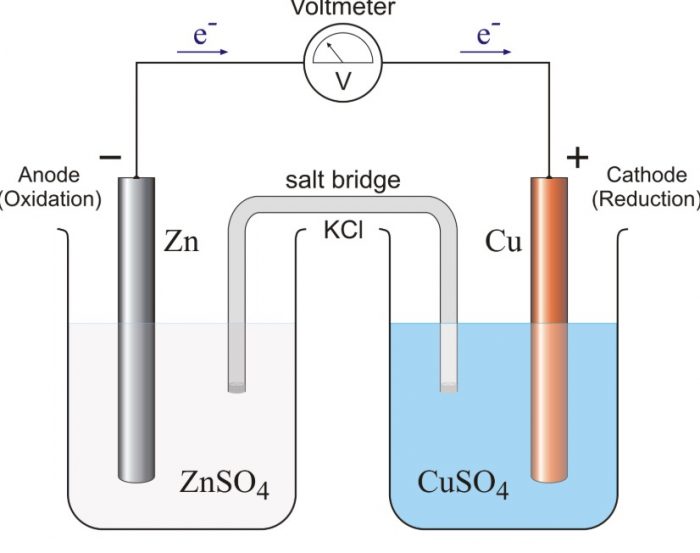
Electrochemical Cell or Galvanic Cell
The devices in which electrical energy is produced from chemical reactions are called electrochemical cells or galvanic cells or voltaic cells.
In these cells, oxidation and reduction reactions occur in separate containers called half cells and the redox reaction is spontaneous. Electrical energy is produced during such reactions.
1) The arrangement consists of two beakers, one of which contains 1.0 M solution of zinc sulphate and the other 1.0 M solution of copper sulphate.
2) A zinc rod is dipped into ZnSO4 solution while a copper rod is dipped into CuSO4 solution. These metallic rods are known as electrodes.
3) The metallic rods in the beaker are connected to the ammeter by means of an insulated wire through a key.
4) Ammeter is used to know the passage of current which moves in opposite direction to the flow of electrons.
5) The solutions in the two beakers are connected by an inverted U-tube containing saturated solution of some electrolyte such as KCl, KNO3, or NH4NO3 which does not undergo a chemical change during the process. The saturatecd solution is generally taken in agar-agar jelly or gelatin.
6) The two openings of the U-tube are plugged with some porous material such as glass wool or cotton. The U-tube which connects the two glass beakers is called a salt-bridge.
7) When the circuit is complete by inserting the key in the circuit, it is observed that electric current flows through external circuit as indicated by the ammeter.
Observation
(1) Zinc rod gradually loses its weight.
(2) The concentration of Zn2+(aq) in the ZnSO4(aq) solution increases.
(3) Copper gets deposited on the electrode.
(4) The concentration of Cu2+(aq) in the CuSO4 (aq) solution decreases.
(5) There is a flow of electrons in the external circuit from zinc rod to copper rod. Therefore, the current flows from copper to zinc.
(6) The flow of electric current is taken opposite to the flow of electrons.
During the reaction, zinc is oxidised to Zn2+ ions which go into the solution. Therefore,
Zn(s) ———> Zn2+ (aq) + 2e¯ (Oxidation)
the zinc rod gradually loses its weight. The electrons released at the zinc electrode move towards the other electrode through outer circuit. Here, these are accepted by Cu2+ ions of CuSO4 solution which are reduced to copper. The metal gets deposited on the copper electrode.
Cu2+ (aq) + 2e¯ ————> Cu(s) (Reduction)
The zinc electrode where electrons are released or oxidation occurs is called anode while the copper electrode where electrons are accepted or reduction occurs is called cathode. As the electrons move from zinc rod to the copper rod, the zinc rod is regarded as negative terminal while copper rod is regarded as positive terminal.
There is flow of electrons from negative terminal (anode) to positive terminal (cathode).
In an electrochemical cell:
1) Oxidation occurs at anode (-ve terminal)
2) Reduction occurs at cathode (+ve terminal)
3) Electrons flow from anode to cathode.
The two containers involving oxidation and reduction half reactions are called half cells.
The zinc rod dipping into a ZnSO4 solution is oxidation half cell and the copper electrode dipping into a CuSO4 solution is reduction half cell.
Salt Bridge and its functions
It is usually an inverted U-tube filled with concentrated solution of inert electrolyte. The essential requirements of electrolyte are:
1) The mobility of the anion and cation of the electrolyte should be almost same.
2) The ions of the electrolyte are not involved in electrochemical change.
3) The ions do not react chemically with the species of the cell. Generally, salts like KCl, KNO3, NH4NO3 are used. The saturated solutions of these electrolytes are prepared in agar agar jelly or gelatin. The jelly keeps the electrolyte in semi-solid phase and thus prevents mixing.
Functions of the salt bridge
1) Salt bridge completes the electrical circuit
The salt bridge connects the two solutions of the half cells and their electrodes are connected by means of a wire.
2) Salt bridge maintains electrical neutrality of two half cell solutions
The electrons released by the oxidation of Zn to Zn2+ ions will be accepted by the Cu2+ ions of CuSO4 in the other half cell and the latter will be reduced to copper. The positively charged Zn2+ ions pass into the solution. After sometimes, this results into accumulation of extra positive charge in the solution around cathode will acquire extra negative charge due to excess of SO42- ions.
The accumulation of positive charge around zinc rod will prevent the further flow of electrons from the zinc rod. Similarly the accumulation of negative charge around copper electrode will prevent the flow of electrons to the copper ions. Thus, the flow of electrons will occur only momentarily and the cell will stop working.
The accumulation of charges in the two half cells is prevented by using salt bridge, which provides a passage for the flow of the charge in the internal circuit. When the concentration of Zn2+ ions around anode increases sufficient number of Cl¯ ions migrate from the salt bridge to the anode half cell.
To neutralise the excess negative charge due to the additional S042- in cathode half cell, sufficient number of K+ ions migrate from the salt bridge to this half cell. Thus, the salt bridge provides cations and anions to replace the ions lost or produced in the two half cells.
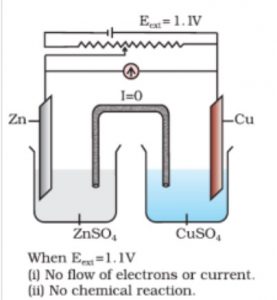
Functioning of the cell when external opposing potential is applied
Consider a Daniel cell in which the zinc electrode is dipped in ZnSO4 (aq) and copper electrode is dipped in CuSO4 (aq) solution.The cell reaction occurring is
Zn (s) + Cu2+ (aq) ⇔ Zn2+ (aq) + Cu (s)
The electrical potential (called emf) of the cell is 1.10 V. When concentration of Zn2+ and Cu2+ ions is 1 M (1 mol d m-3).
Consider an arrangement in which an external opposite potential is applied. Let us assume that the external opposite potential is increased slowly. It is observed that the reaction continues to take place till the opposing voltage reaches the value of 1.1 V.
As long as the external opposite is less than 1.10V, the electron continue to flow from Zn rod to Cu rod and hence current flows from Cu to Zn. Zinc dissolves in anode and copper deposits at cathode.
When the opposing voltage reaches the value 1.10 V, the cell reaction stops altogether and no current flows through the cell.
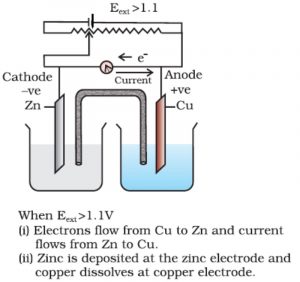
Further increase in the external potential now again starts the reaction but in the opposite direction. In this case electrical energy is used to carry out non-spontaneous reaction. The electrons flow from Cu to Zn rod and current flows from Zn to Cu.
Representation of an Electrochemical Cell
An electrochemical cell or galvanic cell consists of two electrodes: anode and cathode. The electrolyte solution containing these electrodes are called half cells. When these two half cells are combined, a cell is formed.
1) A galvanic cell is represented by writing the anode (where oxidation occurs) on the left hand side and cathode (where reduction occurs) on the right hand side.
2) The anode of the cell is represented by writing metal or solid phase first and then the electrolyte (or cation of the electrolyte) while the cathode is represented by writing the electrolyte first (or cation) and then metal and solid phase.
The metal and the cation are separated either by a semicolon (;) or by a vertical line.The concentration of the electrolyte is also mentioned within bracket after the cation, i.e.
Zn;Zn2+ or Zn | Zn2+ or Zn|Zn2+ (1 M)
Cu2+;Cu or Cu2+ | Cu or Cu2+ (1 M) | Cu
3) The salt bridge which separates the two half cells is indicated by two vertical lines.
For example: Zn | Zn2+ (1M) || Cu2+ (1M) | Cu
1) Sometimes negative and positive signs are also put on the electrodes to show the release and loss of electrons taking place on them.
2) Anode is a negative pole while cathode acts as positive pole.
3) The electrons flow from the negative pole (anode) to the positive pole (cathode) in the external circuit. The current is said to flow in the opposite direction.
Memory Aid
As a memory aid keep in mind the alphabetical order of the first letter e.g., A(anode) comes before C(cathode). The cell may be written by arranging each of the pair left-right, anode- cathode, oxidation-reduction, negative and positive in the alphabetical order as:
Left , Anode, Oxidation, Negative
Right , Cathode, Reduction, Positive
Representation of Some Common Cells by Cell Notation
1) Cu-AgNO3 Cell
Oxidation half reaction: Cu ——>Cu2+ +2e¯
Reduction half reaction : 2 Ag+ + 2e¯ ——-> 2Ag
Overall cell reaction: Cu + 2 Ag+———> Cu2+ + 2Ag
The cell may be represented as:
Cu(s) | Cu2+ (aq) || Ag+ (aq) | Ag(s)
2) Ni-AgNO3 Cell
Oxidation half reaction: Ni(s) ——-> Ni2+ (aq) +2e¯
Reduction half reaction : 2Ag+ (aq) + 2e¯ + 2Ag(s)
Overall cell reaction: Ni(s) + 2Ag+ (aq) ——–> Ni2+ (aq) + 2Ag(s)
Ni l Ni2+(aq) || Ag+(aq) | Ag(s)
3) Zn-HCl Reaction
Oxidation half reaction : Zn(s) ——> Zn2+(aq) + 2e¯
Reduction half reaction: 2H+(aq) + 2e¯ ————> H2(g)
Overall cell reaction:
Zn(s) +2H+(aq) ——–> Zn2+(aq) +H2 (g)
Cell may be represented as:
Zn(s) | Zn2+(aq) || H+(aq) | H2
thanx
Thanks mam
God bless you …
Thank you ma’am.. This really helped me a lot.
Thank-you mam
Thanks for notes