Contents
Enthalpy of Fusion
Enthalpy of fusion is the enthalpy change accompanying the transformation of one mole of a solid substance into its liquid state at its melting point. it is also called molar enthalpy of fusion.
The molar enthalpy of fusion ( Δ fus H ) of ice is +6 KJ mol-1.
H2O ( s ) → H2O ( l ) Δfus H = + 6 KJ mol-1
The enthalpy of freezing has same value as enthalpy of fusion but has the opposite sign.
H2O ( l ) → H2O (s) Δ freezing H = − 6 KJ mol-1
Enthalpy of Vaporisation
It is the amount of heat required to convert 1 mole of a liquid into its vapour state at its boiling point.
It is also called molar enthalpy of vaporisation.
The molar enthalpy of vaporisation of water into its vapour at the boiling point of water is 40.7 KJ.
H2O ( l ) → H2O ( g ) Δ vap H = + 40.7 KJ mol-1
As condensation is reverse of vaporisation, the enthalpy of condensation has the same value as the enthalpy of vaporisation but has opposite sign.
H2O ( g ) → H2O ( l ) Δfus H = − 40.7 KJ mol-1
Enthalpy of Sublimation
Sublimation is a process in which a solid on heating changes directly into gaseous state below its melting point.
Enthalpy of sublimation of a substance is the enthalpy change accompanying the conversion of one mole of a solid directly into vapour phase at a given temperature below its melting point.
The enthalpy of sublimation of iodine is 62.39 KJ mol-1
I2 ( s ) → I2 ( g ) Δ sub H = + 62.39 KJ mol-1
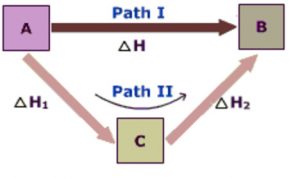
Hess’s law of constant Heat Summation
Hess’s law states that
The total amount of heat evolved or absorbed in a reaction is the same whether the reaction takes place in one step in or in a number of steps. The total amount of heat change in a reaction depends only upon the nature of the initial reactants and the nature of the final products and is independent of the path or the manner by which this change is brought about.
For example:
1) When carbon burns to form carbon dioxide directly in one step 393.5 KJ mol-1 of heat is produced
C ( s ) + O2 ( g ) → CO2 ( g ) Δr H = − 393.5 KJ mol-1
If carbon burns to form carbon dioxide monoxide first which then burns to form carbon dioxide, the heat evolved in the two steps are :
C ( s ) + ½ O2 ( g ) → CO ( g ) ΔrH1 = -110 KJ mol-1
CO ( g ) + ½ O2 ( g ) → CO2 ( g ) ΔrH2 = -283 KJ mol-1
The total heat evolved in the two steps will be ΔH° = -393.5 KJ mol-1
which is same when the reaction take place in one step.
2) Sulphur burns to form SO3 in one step as:
S ( R ) + 3/2 O2 ( g ) → SO3 ( g ) ΔrH° = – 395.4 KJ mol-1
Sulphur may change to SO3 in two steps as follow:
1) S ( R ) + O2 ( g ) → SO2 ( g ) ΔrH1 = – 297.5 KJ mol-1
2) SO2 ( g ) + ½ O2 ( g ) → SO3 ( g ) ΔrH2 = – 97.9 KJ mol-1
Total heat evolved in two steps is -395.4 KJ mol-1.
This is the same as for the direct reaction in one step.
Application of Hess’s law
1) In the calculation of enthalpy of formation
The enthalpies of formation of many compounds cannot be determined experimentally. These are calculated by the application of Hess’s law.
2) Calculation of enthalpy of allotropic transformation
Elements like carbon and sulphur exist in different allotropic forms. The change of one form to other involves a very small amount of heat and is a very slow process.
The experimental determination of heat changes for such transformation is very difficult. These are calculated by application of Hess’s law.
3) Enthalpy of hydration
The experimental determination of the enthalpy of hydration is almost impossible. It can be easily calculated using Hess’s law.
4) Predicting the enthalpy change for any reaction
Hess’s law can be applied to predict the enthalpy change for any reaction from the enthalpy changes of certain other reactions.
Leave a Reply