The distribution of electrons into different shells, sub shells and orbitals of an atom is called its electronic configuration.
The electronic configuration of any orbital can be represented as: nlx
n is the number of principal shell, l = symbol of the sub shell or orbital, x= number of electrons present in the orbital
4p1 means that p- sub shell of the 4th main shell contain one electron.
In certain elements when the two sub shells differ slightly in their energies, an electron may shift from a sub shell of lower energy to a sub shell of higher energy only if such a shift results in the symmetrical distribution of the electrons in the various orbitals of the sub shell of higher energy.
Symmetrical distribution: The electronic configuration in which all the orbitals of the same sub shell are either completely filled or are exactly half filled are more stable because of symmetrical distribution of electrons.
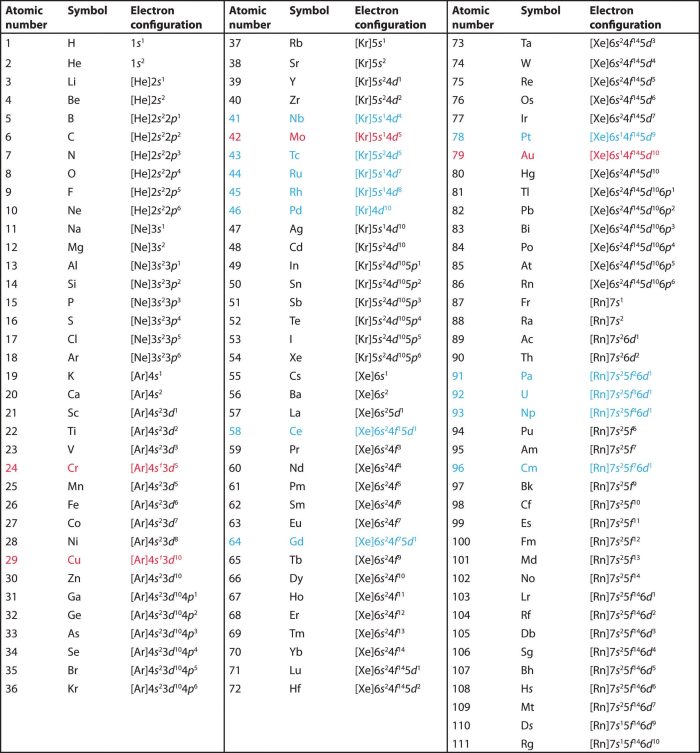
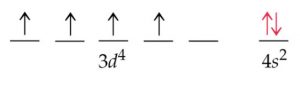
1) The expected electronic configuration of chromium
If one of the 4s electron shifts to the vacant 3d orbital ,the distribution of the electron will become more symmetrical and this will impart extra stability.
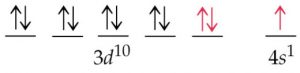
The actual electronic configuration of chromium
2) The expected electronic configuration of copper
If one of the 4s electron shifts to the vacant 3d orbital ,the distribution of the electron will become more symmetrical and this will impart extra stability.
The actual electronic configuration of copper
Exchange energy
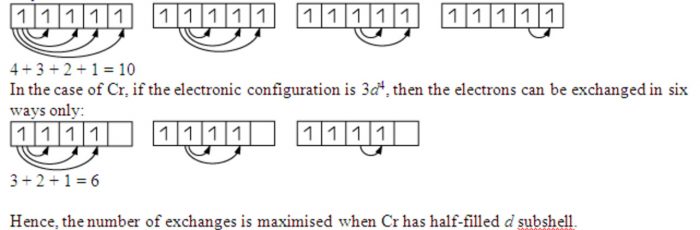
The electrons with parallel spins present in the degenerate orbitals tend to exchange their position .The energy released during this exchange is called exchange energy.
The number of exchanges that can take place is maximum when degenerate orbitals are exactly half filled or completely filled.As a result, the exchange is maximum and so is the stability.
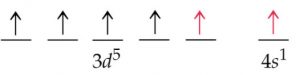
I get a great help from this.That’s why its good….
Thanks
Mam
How can I get soft copy of these notes in word file…
pls tell me the cost..Pls Contact
Hi Sanjay, Thanks for reaching us, right now we do not provide soft copy in word file, we are working on making pdf files that would be available on the site very soon.
Bohot mast
It was so much helpful thank you soooooo much
It was too much helpful
I am very very glad to see such a arranged notes, mam I really appreciate your this many effort and also thanking you for your help which I can’t find on any other platforms. your notes are the best.