Some liquids like water, ether flow rapidly why some other liquids like glycerine, castor oil flow quite slowly.
This internal resistance to flow possessed by a liquid is called its viscosity
The liquids which flow slowly, have high internal resistance which is due to strong intermolecular forces and therefore, are said to be more viscous or are said to have high viscosity.
The liquids which flow rapidly have low internal resistance which is due to weak intermolecular forces and hence are said to be less viscous or said to have low viscosity.
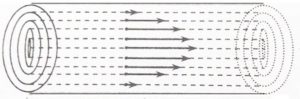
Consider a liquid flowing through a narrow tube. All parts of the liquids do not move through the tube with same velocity. Imagine the liquid to be made up of a large number of thin cylindrical coaxial layers. The layers which is in contact with the walls of the tube is almost stationary. As we move from the wall towards the centre of the tube, the velocity of the cylindrical layers keeps on increasing till it is maximum at the centre.
This type of flow in which there is a regular gradation of velocity in going from one layer to next is called laminar
As we move from centre towards the walls, the velocity of the layers keep on decreasing. In other words, every layer offers some resistance or friction to the layer immediately below it.
This force of friction which one part of the liquid offers to another part of the liquid is called viscosity.
The force of friction f between two layers each having area A sq cm, separated by a distance dx cm, and having a velocity difference of dv cm/sec, is given by,
f ∝ A ( dv / dx )
f = η A ( dv/dx)
where η is a constant known as coefficient of viscosity.
dv/dx is called velocity gradient.
If dx =1, A = 1 sq cm
dv = 1 cm/sec
then
f = η
Coefficient of viscosity may be defined as the force of friction required to maintain a velocity difference of 1 cm/sec between two parallel layers, 1 cm apart and each having an area of 1 sq cm.
Some important results
1) Units of viscosity
η = f .dx / A .dv
η = dynes × cm / cm2 ×cm/sec
η = dynes cm-2 sec
The units of viscosity are dynes sec cm-2.This quantity is called 1 Poise.
f = m × a
η = (m × a × dx) / ( A .dv)
η = g cm-1 s-1
η = 1 poise
In S.I. units
η = f .dx / A .dv
η = N × m / ( m2 ×ms-1)
η = N m-2 or Pa s
1 poise = 1 g cm -1 s-1 = 0.1 kg m-1 s-1
2) Effect of nature of the liquid on viscosity
Greater are the intermolecular forces, higher is the viscosity of the liquid. Water has higher viscosity than methyl alcohol because intermolecular forces in water are greater than those in methyl alcohol.
Hydrogen bonding and van der waals forces are strong enough to result into high viscosity of the liquid.
3) Effect of temperature on viscosity
With increase in temperature, the kinetic energy of the molecules of the liquid increases which can overcome the intermolecular forces. Hence the liquid starts flowing faster. The viscosity of a liquid decreases with increase in temperature.
It is very useful
Thanks it is too important
It is very helpful for us to understand the portion in effective manner
Thanks alot to provide this notes
We can easly understand the concept by
this notes
Thanks……
Vry helpful……..!!!!