The liquefaction of a gas takes place when the intermolecular forces of attraction become so high that they bind the gas molecules together to form the liquid state.
The intermolecular forces of attraction can be increased either by increasing the pressure so that the molecules come close together or by cooling the gas so that the kinetic energy of the molecules decreases and they become slower.
The effect of temperature on the liquefaction of gases is found to be very important as higher the temperature of the gas ,more difficult it is to liquefy it and higher is the pressure required.
Gas like hydrogen ,helium, oxygen, nitrogen could not be liquefied at room temperature by application of pressure alone. Each of these gases could also be liquefied provided first it is cooled down to or below a particular temperature. For each gas, there is a particular temperature above which it cannot be liquefied, howsoever, high pressure may be applied on the gas. This temperature is called critical temperature.
Critical temperature of a gas may be defined as that temperature above which it cannot be liquefied however high pressure may be applied on the gas.
The pressure required to liquefy the gas at the critical temperature is called critical pressure .
The volume occupied by 1 mole of the gas at critical temperature and critical pressure is called critical volume.
All the three are called critical constants of the gas and are represented by Tc, Tp, Tv.
Andrews experiment on critical phenomena
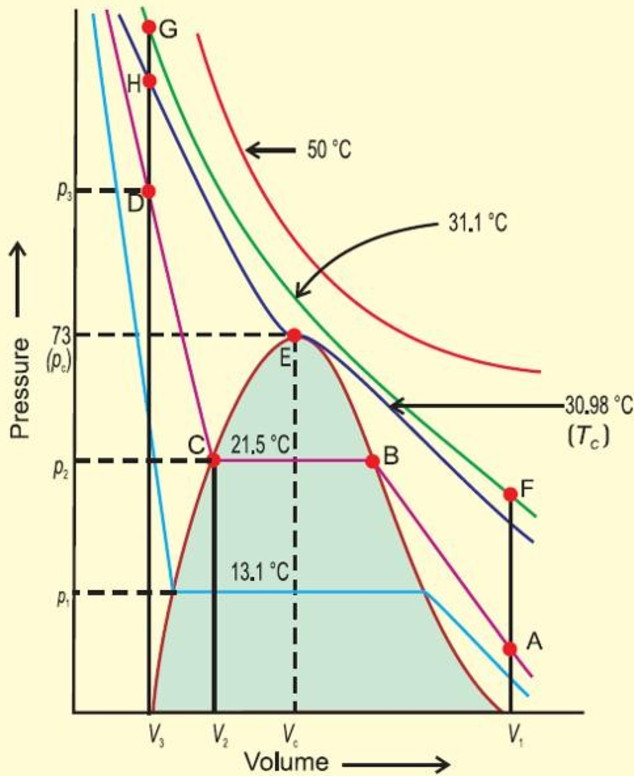
Andrews in 1861 was the first to study the critical phenomena experimentally using carbon dioxide gas.
He studied the effect of pressure on the volume of carbon dioxide at different constant temperature. The plots obtained are called isotherms.
At the lowest temperature employed i.e. 13.1° C , at low pressure, carbon dioxide exist as a gas, as shown at the point A.
As the pressure is increased, the volume of the gas decreases along the curve.
The point at which liquefaction of the gas starts, volume decreases rapidly because liquid has much less volume than the gas.
Once the liquification is complete, the increase in pressure has very little effect upon volume because liquids are very little compressible. Hence a steep curve is obtained.
As the temperature is increased, horizontal portion becomes smaller and smaller and at 30.98 °C , it is reduced to a point E.
Above 30.98°C ,the gas cannot be liquefied at all, however high pressure may be applied. Thus 30.98°C is the critical temperature.
We get a dome shaped curve.
A point like A represent gaseous state.
A point Like D represents a liquid state.
A point within the dome shaped area represents existence of liquid and gaseous carbon dioxide in equilibrium.
Importance of critical temperature
The critical temperature of a gas is a measure of the strength of the intermolecular forces of attraction.
Weaker are the intermolecular forces ,more difficult it is to liquefy that gas and hence lower would be the critical temperature of that gas.
Helium and hydrogen have weak intermolecular forces, thus they are difficult to liquefy and hence have low critical temperature.
Carbon dioxide and ammonia have strong intramolecular forces of attraction, they can be easily liquefied and their critical temperature are high which are above room temperature.
Van der waals constant a is also a measure of intermolecular forces of attraction. Hence it is found that the values of constant a increase in the same order as the critical temperature.
As a gas can be liquefied below the critical temperature by applying pressure. Therefore above the critical temperature ,it is a gas but below the critical temperature it is a vapour.
For ex: Carbon dioxide below its critical temperature is called carbon dioxide vapour.
This site is the most useful resource I’ve found online, hats off to Mrs. Shilpi Nagpal for her efforts. Keep up the excellent work ma’am, you make us students want to read the subject.
thanks sunny for this wonderful comment.
this site is very very very useful for me.
Thanks asish
This site is important for self study
Nice explanation.
This ineed was vry useful and self expalanatory so thanks for such explanation
Thanks for this wonder explanation. ALL DOUBTS GOT CLEARED AT ONCE. The best way it could have been explained is this. Thanks alot Shilpi Nagpal Ji.
Thanks! Rishabh for appreciating, please keep visiting our website and share this educational resource with others as well.
Tanks for such a wonderful note given by Mrs. Shilpi Nagpal
Explanations are very clear. Thank u shilpi man
This site is very good for self study.
Thank you for the explanation of the topic.
Wonderful explanation…