Characteristics of Gases
1) Gases have neither definite shape nor definite volume. They take up the shape and volume of the container.
2) They have lower density than liquids and solids.
3) They are highly compressible
4) Gases intermix completely in all proportion without any mechanical aid.
5) They exert pressure equally in all direction.
Measurement of mass
The mass of a gas can be easily determined by weighing the container containing the gas, and then emptying the container by taking out the gas and weighing the empty container again. The difference between the two masses gives the mass of the gas.
1 mole= 6.022 × 1023
Measurement of volume
As a gas fills the whole of the vessel in which it is put, hence the volume of the gas is equal to the volume of its container which in turn can be calculated from the dimensions of the container.
The S.I. unit of volume is m3.
1 m3 = 103 dm3 = 106 cm3
1 ml =1 cm3
1 L = 103 cm3 =1 dm3 = 10-3 m3
Measurement of pressure
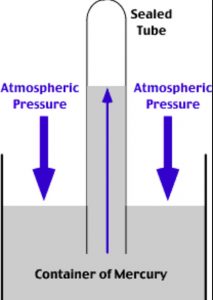
The instrument used for the measurement of atmospheric pressure is called a barometer.
It consists of inverting a tube filled with mercury in a dish of mercury. The height of the mercury column above the level of mercury in the dish is a measure atmospheric pressure at that place.
Mercury is used as a barometric liquid because
1) The height of the column in a barometer is inversely proportional to the density of the liquid. As Mercury has very high density, the height of the column setup is very convenient for study.
2) Mercury is non-volatile at room temperature. Hence, the vapour pressure due to Mercury vapour is negligible.
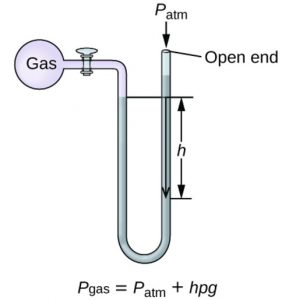
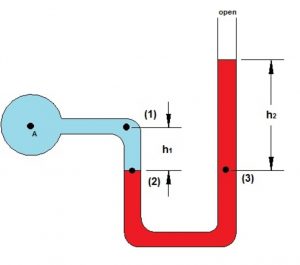
The instrument used for the measurement of the pressure of a gas is called manometer. It simply consists of a U- shaped tube containing mercury usually.
Two types of manometers are:
1)Those in which the longer limb is closed. Closed limb manometer is used only for gases at pressure less than the atmospheric pressure.
2)Those in which the longer limit is open.Open limb manometer is used for all.
Suppose height of the mercury column= h cm
Area of cross section of the tube= A cm2
Volume of the mercury column= A × h cm3
Density of mercury at room temperature= ρ g cm-3
mass of mercury column=A × h × ρ grams
weight of mercury column= (A × h × ρ ) × g gram
Weight of mercury column is the force acting on A cm3.Hence
Pressure= h × ρ × g
A standard or normal atmospheric pressure is defined as the pressure exerted by a mercury column of exactly 76 cm at 0°C. This is the pressure exerted by the atmosphere at the sea level.
1 atm= 76 cm = 760 mm = 760 torr
1 atm= 1.01325 bar
1atm= 0.987 atm
The S.I. unit of pressure is pascal(Pa)
1 Pa= 1 Nm-2 = 1kg m-1 s-2
1atm= 1.01325 × 105 Nm-2
1 bar= 102 kPa
Measurement of temperature
Temperature is a measure of the extent of hotness or coldness of a body.
It is based on the principle that substances expands on heating.
Substance used in the measurement of temperature is mercury.
There are three different scales on which the temperature are measured
1)Fahrenheit scale
2)Kelvin scale
3)Celsius scale
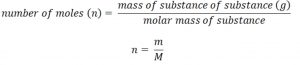
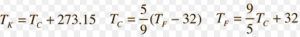
Leave a Reply