A solution is defined as a homogeneous mixture of two or more chemically non reacting substances, the relative amount of which can be varied up to a certain limit.
If a solution consist of only two components it is called a binary solution.
The component present in smaller amount is called solute while the other present in larger amount is called solvent
The concentration of the solution can be expressed in number of ways:
(1) Mass percent or weight percent :Mass percent of a solute in a solution is the mass of the solute in grams present in 100 grams of solution.
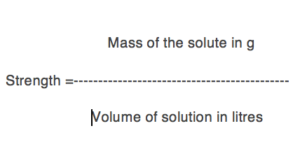
(2) Strength: Strength of a solution is defined as the amount of the solute in grams present per litre of the solution.
(3) Molarity: Molarity of a solution is defined as the number of moles of the solute present per litre of the solution.It is represented by the symbol, M.
(4) Molality :The molality of a solution is defined as number of moles of the solute dissolved in 1 kg of the solvent.
It is represented by the symbol m.
(5) Normality : Normality of a solution is defined as the number of gram equivalent of the solute present per litre of the solution.
It is represented by the symbol N.
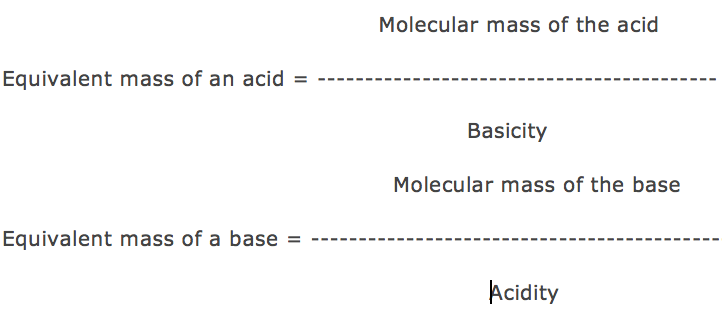
Basicity is the number of displaceable H+ ions present in 1 molecule of the acid.
Acidity is the number of displaceable OH– ions present in 1 molecule of the base.
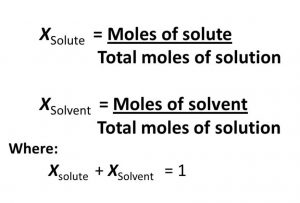
(6) Mole fraction : Mole fraction of any component in the solution is equal to the number of moles of that component divided by the total number of moles of all the components.
For a solution containing n2 moles of the solute dissolved in n1 moles of the solvent,
(7) Parts per million: The concentration of very dilute solution is expressed in terms of parts of the solute by mass present in million parts by mass of the solution.
Molarity equation
If a solution having molarity M1 and volume V1 is diluted to volume V2 so that the new molarity is M2 then as the total number of moles in the solution remains the same, we have :
Normality Equation
Relation between molarity and normality of the solution of an acid or base
Normality of solution of an acid = Molarity × Basicity
Normality of solution of a base = Molarity × Acidity
Calculation of Molarity and Normality of the solution obtained on mixing two or more solution
If the solutions mixed are of the same substance
If V1 mL of a solution of molarity M1 are mixed with V2 mL solution of the same substance with molarity M2 Or V1 mL of M1 HCL solution are mixed with V2 mL of M2 , then the molarity of the solution obtained is calculates as:
M1 V1 + M2 V2 = M3 (V1 +V2)
N1V1 + N2V2 = N3 (V1 +V2)
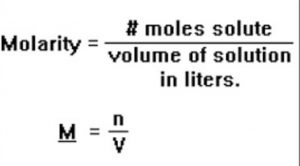
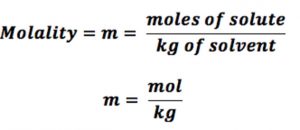

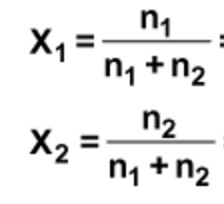
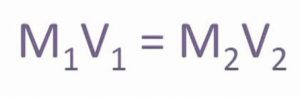
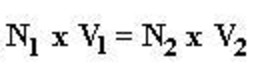
Bhot badiya sirg
Very helpful.
A hearty thanks to you.
it’s really a very good deed . it is really helpful
Thank You
Thank you mam
Thanks Mam for the explanation