Contents
Calculation of Percentage Composition
The percentage of any element or constituent in a compound is the number of parts by mass of that element or constituent present in 100 parts by mass of the compound.
Step 1 : Calculate the molecular mass of the compound from its formula by adding the atomic masses of the element present.
Step 2 : Calculate the percentage of elements or the constituents by applying the relation:
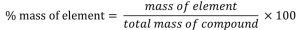
Question : Calculate the percentage of Carbon, Hydrogen and oxygen in Ethanol?
Answer

Empirical and Molecular Formula
The empirical formula of a compound is the chemical formula which expresses the simplest whole number ratio of the atoms of the various elements present in one molecule of the compound.
For Example : The empirical formula of benzene is CH, hydrogen peroxide is HO, Glucose is CH2O.
The empirical formula represents only the atomic ratio of the various elements present in its molecule.
The molecular Formula of a compound is the chemical formula which represents the true formula of its molecules. It expresses the actual number of atoms of various elements present in one molecule of the compound.
The molecular formula of benzene is C6H6 , hydrogen peroxide is H2O2 , Glucose is C6H6.
Molecular Formula = n × Empirical formula
where n is any integer such as 1, 2, 3 ,4 etc.

The value of n can be obtained from the relation:
Molecular mass = 2 × Vapour Density
Calculation of Empirical Formula
Step 1 : Convert the mass percentage into grams.
Step 2 : Calculate the number of moles.

Step 3 : Calculate the simplest molar ratio: Divide the moles obtained in step 1 by the smallest quotient or the least value from amongst the values obtained for each element.
Step 4 : Calculate the simplest whole number ratio.
Step 5 : Write the empirical formula.
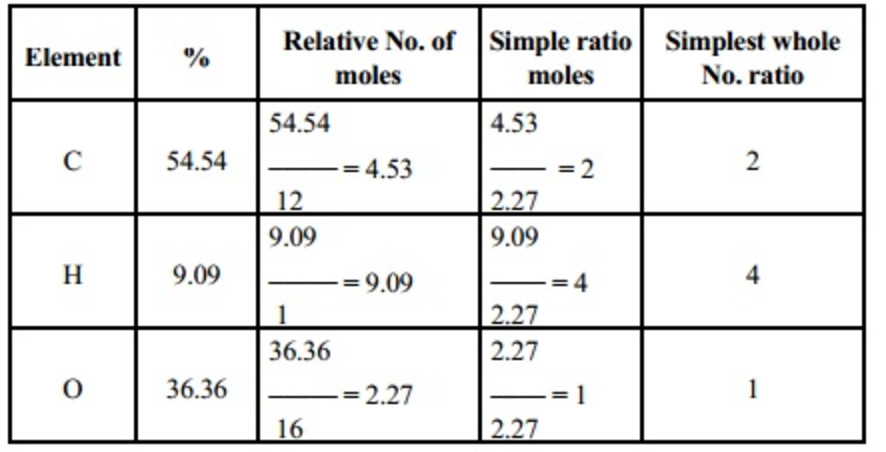
The Empirical Formula is C2H4O.
Calculation of molecular Formula
The molecular formula of a compound can de deduced from:
1) Empirical Formula
2) Molecular mass
Step 1 Calculation of the empirical formula from the percentage composition.
Step 2 Calculation of empirical formula mass by adding the atomic mass of all the atoms present in the empirical formula.
Step 3 Determination of the molecular mass of the compound from the given data.
Step 4 Determination of the value of n.
Step 5 Determination of the molecular formula
Question A compound contains 4.07 % hydrogen, 24.27 % carbon and 71.65 % chlorine. Its molar mass is 98.96 g. What are its empirical and molecular formula.
Answer
| Element | Symbol | Percentage of Element | Atomic Mass |
Moles of Element | Simplest Molar Ratio | Simplest whole no. Molar ratio |
| Carbon | C | 24.27 | 12 | 2.02 | 1 | 1 |
| Hydrogen | H | 4.07 | 1 | 4.07 | 2 | 2 |
| Chlorine | Cl | 71.65 | 35.5 | 2.02 | 2 | 1 |
The empirical formula of the compound is CH2ClEmpirical mass of CH2Cl = 12 + 2 × 1 +35.5 = 49.5
n=2
Molecular formula = n × E. F.
= 2 × CH2Cl
=C2H4Cl2
Nice
Thanks
Really nice
Thanks for this data . I was finding everywhere but could not found but here I got the details …… Thanks ..
Good explanation
thank you
Really super it was so helpful. Thank u so much
complete and detailed explanation
Really nice I understand
Nice but would have been more better if it involved more questions other than NCERT examples