Alkali metals cannot be extracted from their ores by the usual methods of extraction of metals because of following difficulties:
1) Alkali metals are strong reducing agents and hence cannot be extracted by reduction of their oxides or chlorides.
2) Alkali metals being highly electropositive cannot be displaced from the aqueous solution of their salts by other metals.
3) Alkali metals cannot be isolated by electrolysis of the aqueous solution of their salts since hydrogen is liberated at the cathode instead of the alkali metal because the standard electrode potentials of alkali metals are much lower than that of water. By using mercury as cathode ,the alkali metals can be deposited at the cathode but the alkali metals so deposited readily combines with mercury to form an amalgam from which its recovery is very difficult.
Extraction of Lithium
Lithium is prepared by electrolysis of fused mixture of dry lithium chloride and potassium chloride at 723 k.
Potassium chloride is added to increase the conductivity of lithium chloride and also to lower the fusion temperature. The cell is operated at a temperature of about 723 K and current voltage of 8-9 V.
As a result of electrolysis, the following reactions takes place:
LiCl ⇔ Li+ + Cl‾
At cathode: Li+ + e‾ ——-> Li
At anode: 2 Cl‾ – 2e‾ —–> Cl2
The metal thus obtained is 99% pure and is stored by keeping it wrapped in paraffin wax. Lithium being the lightest metal known cannot be stored in kerosene oil since it floats on the surface.
Uses of Lithium
1) It is used in the manufacture of alloys
a) Lithium – lead alloy or white metal which is used for making toughened bearing for motor engines and sheats for cables.
b) Lithium aluminium alloy has great tensile strength and electricity like that of mild steel. It is used for aircraft construction.
c) Lithium magnesium alloy is extremely tough and corrosion resistant which is used for armour plate and aerospace components.
2) It is used for producing thermonuclear energy required for propelling rockets and guided missiles.
3) Lithium is used to make both primary and secondary batteries.
4) Lithium is used as a Scavenger since it combines readily with oxygen and nitrogen. Thus it is used for removing little traces of oxygen and nitrogen during refining of metals.
5) lithium carbonate is used in making a special variety of glass which is very strong and is weather proof.
6) Lithium chloride is used in Air conditioning plants to regulate the humidity. It is also used in Ni-Fe accumulators.
7) Lithium Bromide is used in medicine as sedative.
8) Lithium bicarbonate and lithium salicylate have been used for treatment of rheumatism since the resulting Lithium urinate is soluble in water.
9) Lithium hydride is used as source of hydrogen for meterological purpose and for filling of balloons.
10) Lithium Hydroxide is used for removing carbon dioxide from exhaled air in closed quarters.
11) Lithium aluminium hydride is used as a reducing agent in synthetic organic chemistry.
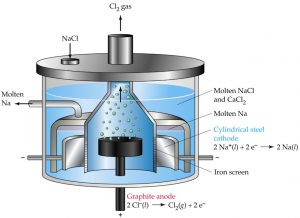
Extraction of Sodium
Sodium is extracted by the electrolysis of fused sodium chloride by a process called Down’s process.
Sodium is now obtained by electrolysis of a fused eutectic mixture of sodium chloride and calcium chloride in Down’s cell at 873 K using graphite anode and iron cathodes.
As a result of electrolysis ,sodium is liberated at the cathode and Cl2 is evolved at the anode.
NaCl ——–> Na+ + Cl‾
At cathode: Na+ + e‾ ———> Na
At anode: Cl‾ ———-> Cl + e‾
Cl + Cl ——-> Cl2
Uses of Sodium
1) Sodium is used as a reducing agent in the extraction of boron and silicon.
2) Sodium is employed as a reducing agent in form of sodium amalgam and as a reagent in Wurtz reaction and in the synthesis of many organic compound.
3) It is also used to make tetraethyl lead, tetra methyl lead which are used as anti knocking agent for gasoline.
4) liquid Na or its alloys with potassium is used as a coolant in fast breeder nuclear reactor.
5) It is used in the manufacture of number of chemicals such as Na2O2 , NaCN, NaNH2.
6) It is used in sodium vapour lamp.
7) Sodium is largely used in industry for the production of artificial rubber , dyes, drugs.
8) Because of its lightness and high thermal conductivity, it is used for filling exhaust valves of aeroplane engines.
Use of Potassium
1) It has a vital role in biological system.
2) KCl is used as fertilizer.
3) KOH is used in manufacture of soap.
4) It is also used as an excellent absorbent for Carbon dioxide.
I got a very useful chemical information here. Thanks
Useful information in a systematic way is provided here.
Thanks!
good but I wanted it in general, like the general extraction if that’s possible.
Thanks though
Useful info….
Thank you!