Contents
Physical Properties
Physical Properties are those which can be measured or observed without changing the identity or composition of the substance.
For example : Mass, volume, melting point, boiling point.
Chemical properties
Chemical properties are those in which a chemical change in the substance occurs.
The measurement of any physical quantity consists of two parts:
(a)The number
(b)The unit
For example: If an object weighs 4.5 kg it involves two parts:
4.5 is the number and kg is the unit.
A unit is defined as the standard of reference chosen to measure any physical quantity.
French Academy of science in 1791 introduced a new system of measurement called metric system in which the different units of a physical quantity are related to each others as multiples of powers of 10.
For example: The improve System of units has been accepted internationally and is called International
System of units or in short SI units (Systeme Internationale in French).
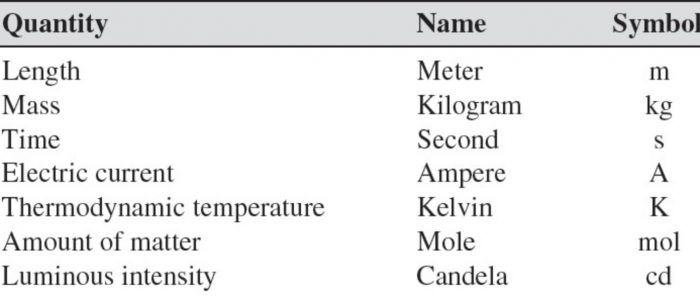
Seven base units
The seven basic physical quantities on which the international System of units is based, their symbols, the name of their units and the symbols of these units are given as:
Measurement of Temperature
There are 3 scales of temperature:
(1) Degree Celsius
(2) Degree Fahrenheit
(3) Kelvin
The SI unit of Temperature is Kelvin
Relation between Degree kelvin and degree Celsius
Relation between degree celsius and Degree Fahrenheit
Measurement of Volume
The SI unit of volume is m³
1 L =1000 mL =1000 cm³
1 L = 1 dm³
1 m³ = 100 (cm)³ = 10³ L
Measurement of Mass
Mass is the quantity of matter contained in the sample and for the given sample it is constant and it does not depend upon the place.
weight is the force with with which the body is attracted towards the earth. It depends upon the acceleration due to gravity which varies from place to place. The mass of a substance can be determined very accurately in the laboratory by using analytical balance or electrical balance.
S.I. unit of mass is kilogram
1 Kg =1000 g
Units of Length
The S. I. unit of length is metre. It is also expressed in angstroms or nanometres or picometres.
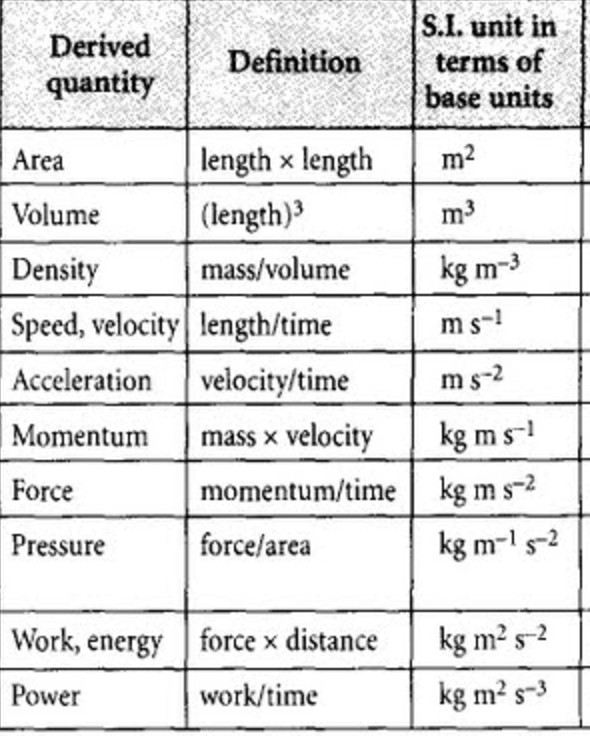
Commonly used physical quantities and their derived units
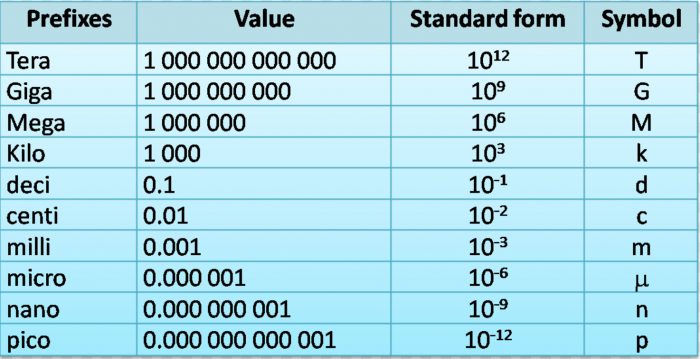
Commonly used prefixes with the base units
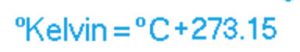
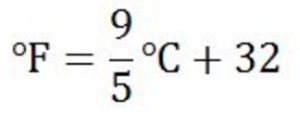
Leave a Reply