Contents
Preparation of Boric Acid
1) From Borax
Boric acid is obtained by treating a hot concentrated solution of borax with hydrochloric acid or sulphuric acid. The resulting solution on concentration and cooling gives crystals of boric acid.
Na2B4O7 + 2 HCl + 5 H2O → 4 H3BO3 + 2 NaCl
Na2B4O7 + H2SO4 + 5 H2O → 4 H3BO3 + 2 Na2SO4
2) By hydrolysis of boron compounds
Boric acid can also be prepared by the hydrolysis of boron compounds such as halides, hydrides and nitrides.
BCl3 + 3 H2O → H3BO3 + 3 HCl
B2H6 + 6 H2O → 2H3BO3 + 6 H2
BN + 3 H2O → H3BO3 + NH3
3) From Colemanite
Boric acid is obtained by passing sulphur dioxide through the solution of the mineral colemanite in boiling water. The resulting solution on concentration and cooling gives crystals of boric acid while calcium bisulphite being highly soluble in water remains in the mother liquid.
Ca2B6O11 + 11H2O → 2 Ca(OH)2 + 6 H3BO3
2 Ca(OH)2 + 4 SO2 → 2 Ca(HSO3)2
–———————————————————————
Ca2B6O11 + 11H2O + 4 SO2 → 2 Ca(HSO3)2
Properties of Boric Acid
1) It is a white crystalline solid with a soft soapy touch having a low density of 1.48 gcm–3.
2) It is sparingly soluble in cold water but fairly soluble in hot water.
3) Acidic Nature
Boric acid behaves as a very weak monobasic acid. It does not act as a proton-donor i.e. protonic acid. Due to the small size of Boron and presence of only 6 electrons in its valence shell , B(OH)3 behaves as a Lewis acid, by accepting a pair of electrons from OH‾ ion of water thereby releasing a proton.
4) Action of Heat
Boric acid ,on heating, loses water in three different stages at different temperature ultimately giving boron trioxide.
H3BO3 → HBO2 + H2O
4 HBO2 → H2B4O7 → 2 B2O3 + H2O
5) Reaction with Ethyl Alcohol
Orthoboric acid reacts with ethyl alcohol in presence of to form conc H2SO4 to form triethylborate.
B(OH)3 + 3 C2H5OH → B(OC2H5)3 + 3 H2O
The vapours of triethyl borate when ignited burns with a green edged flame. This forms the basis for detecting borates and boric acid in qualitative analysis.
Uses of Boric Acid
Boric acid is used
1) in the manufacture of heat resistant borosilicate glass.
2) as a preservative for milk and food stuffs.
3) in the manufacture of enamel and glazes in pottery.
4) the aqueous solution of boric acid is used as a mild antiseptic especially as eye wash under the name boric lotion.
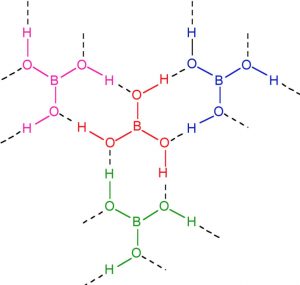
Structure of Boric Acid
The ground state outer electronic configuration of Boron is 2s2 2px1
In excited state, one of the 2s electron gets promoted to the vacant orbital 2py orbital. The three half filled atomic orbitals thus obtained undergo hybridization to give sp2 hybridised orbitals. Each one of these three sp2 orbital overlap with 2p orbitals of O‾ forming 3 B-O‾ bonds.
In boric acid, planar BO33- units are joined by unsymmetrical hydrogen bonds to give a layered structure. The adjacent layers in the crystal of boric acid are held together by weak forces of attraction. The distance between any two successive layer is 318 pm.the Because of weak forces of attraction, one layer can slide over the other. This makes boric acid soft and Soapy to touch.
Thanks mam..
Very useful for me my students