Contents
- 1 Qualitative Analysis
- 2 Procedure for detection of Carbon and Nitrogen
- 3 Modifications in the estimation of Carbon and Hydrogen
- 4 Detection of Nitrogen
- 5 Detection of Halogens
- 6 Preparation of the Lassaigne’s extract
- 7 Function of Nitric Acid
- 8 Detection of Sulphur
- 9 Detection of Phosphorus
- 10 Detection of Oxygen
Qualitative Analysis
Qualitative analysis means to detect the various elements present in it. The elements which commonly occur in organic compounds are carbon, hydrogen, oxygen and nitrogen.
Detection of Carbon and Hydrogen
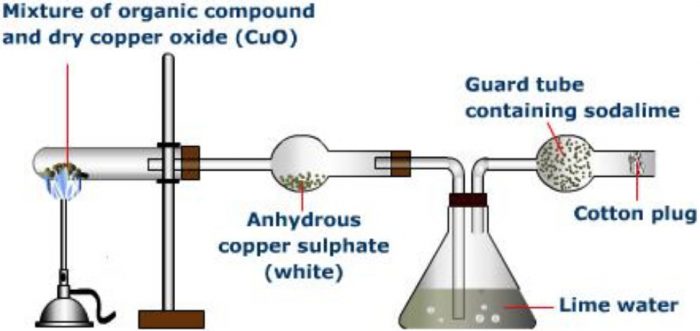
Principle: The presence of carbon and hydrogen, in an organic compound, is detected by heating the given compound with dry Copper(II) oxide or cupric oxide in a hard glass test tube when carbon present is oxidised to carbon dioxide and hydrogen is oxidised to water.
C +2 CuO —-> CO2 + 2 Cu
2H + CuO ———-> H2O + Cu
In general, if the organic compound containing only C and H has the molecular formula C,H, then the complete combustion equation may be written as
CxHy +(2 x + y/2) CuO——–> xCO2 + y/2 H2O+ (2x + y/2)Cu
Carbon dioxide turns lime water milky while water condenses on the cooler parts of the test tube and turns anhydrous copper sulphate blue.
Ca(OH)2 + CO2 ——-> CaCO3 + H2O
CuSO4 + 5 H2O ———> CuSO4.5H2O
Procedure for detection of Carbon and Nitrogen
A small quantity of the pure and dry compound is mixed with nearly five to six times its weight of dry and pure cupric oxide powder. The intimate mixture is strongly heated in a hard glass test tube fitted with a delivery tube having a bulb in the centre. The other end of the delivery tube is dipped in lime water taken in a test tube. The bulb in the delivery tube is packed with anhydrous copper sulphate supported over glass wool. On heating, the carbon is oxidised to CO2 which turns lime water milky and hydrogen is oxidised to water which turns anhydrous copper sulphate blue.
Modifications in the estimation of Carbon and Hydrogen
(1) If the organic substance is a volatile liquid or a gas, the vapours of the compound are passed through heated cupric oxide taken in a hard glass test tube and gases evolved are tested for CO2 and H2O vapours.
(2) If the organic compound also contains sulphur then it is oxidised to sulphur dioxide which also turns lime water milky due to the formation of insoluble calcium sulphite.
4CuO + S ——-> 2 Cu2O + SO2
Ca(OH)2 + SO2 ———> CaSO3 + H2O
The out coming gases are first passed through an acidified solution of potassium dichromate which absorbs sulphur dioxide and turns it green, and then through lime water.
Detection of Nitrogen
The presence of nitrogen is detected by the following tests:
1. Dry heating test. If the organic compound containing nitrogen is heated strongly, it gives a smell of burning hair.
Limitation. This test is, however, not reliable since many compounds containing nitrogen do not give this test.
2. Soda lime test. A pinch of the organic compound is heated strongly in a dry test tube with soda-lime(CaO+ NaOH).A smell of ammonia indicates the presence of nitrogen.
NH2CONH2 + 2 NaOH ——–> 2NH3 + Na2CO3
Limitation. This test is also not reliable since many organic compounds containing nitrogen such nitro , azo groups, etc. do not give this test.
3. Lassaigne’s test. In this test, the elements present in the organic compound are converted from covalent form into the ionic form by fusing the compound with sodium metal. This test is carried out as follows:
(1) Preparation of the Lassaigne’s extract. A small piece of freshly cut sodium is heated gently in a fusion tube till it forms a shiny globule.The tube is removed from the flame and a small amount of the organic compound (50-60 mg) is added and the tube heated strongly till it becomes red hot (2–3 minutes). The hot tube is then plunged into a china dish containing 10 – 15 ml. of distilled water. The contents of the china dish are boiled for a few minutes, cooled and then filtered. The filtrate is called Lassaigne’s extract or sodium fusion extract.
(a) Test for Nitrogen. The Lassaigne’s extract is alkaline since the excess of sodium react with water to produce sodium hydroxide. If not, it is made alkaline by adding a few drops of a dilute solution of sodium hydroxide. To a part of this alkaline solution is added a few drops of a freshly prepared solution of ferrous sulphate. The contents are warmed a little, cooled and then acidified with dil H2SO4 .
Appearance of a green or blue colouration indicates the presence of nitrogen. However, appearance of blood colour indicates the presence of both Nitrogen and sulphur.
Chemistry of the test. During fusion, carbon and nitrogen of the organic compound combine to form sodium cyanide.
Na + C + N ———-> NaCN
On heating with ferrous sulphate solution, sodium ferrocyanide. i.e.,sodium hexacyanoferrate(II) is formed and at the same time some ferrous (Fe2+) ions are oxidised to ferric (Fe3+)ions. These Fe3+ ions then react then react with sodium hexacyanoferrate to produce iron (III) hexacyanoferrate which is prussion blue in colour.
2NaCN + FeSO4 ———> Na2SO4 + Fe(CN)2
Fe(CN)2 + 4 NaCN ——-> Na4[Fe(CN)6]
3Na4[Fe(CN)6] +4 Fe3+ ———-> Fe4[Fe(CN)6]3 + 12 Na+
If nitrogen and sulphur both are present in the organic compound, they may combine during fusion to form sodium thiocyanate (sulphocyanide) due to insufficient sodium. This when heated with ferrous sulphate a blood red colouration due to ferric thiocyanate (or sulphocyanide) by reaction with ferric ions is formed by oxidation of ferrous ions.
Na + C + S + N —–> NaSCN
Fe3+ + 3 NaSCN———-> Fe(SCN)3 + 3Na+
But the absence of blood red colouration does not necessarily mean that sulphur is absent. This is due to the reason that in presence of excess of sodium metal, sodium thiocyanate decomposes to form sodium cyanide and sodium sulphide
2Na + NaSCN ——-> Na2S+ NaCN
Detection of Halogens
The presence of halogens in an organic compound is detected by the following tests:
(1) Beilstein test: In this test, a clean and stout copper wire is heated in the non-luminous flame of the Bunsen burner until it ceases to impart any green or bluish green colour to the flame. The heated end is then dipped in the organic compound and again introduced into the Bunsen flame. The appearance of a green or bluish flame due to the formation of volatile cupric halides indicates the presence of halogens in the organic compounds.
Limitations
(1) Organic compounds like urea, thiourea, etc. which do not contain halogens also give this due to the formation of volatile cupric cyanide.
(2) It not tell as to which halogen (chlorine, bromine, or iodine) is actually present in the organic compound.
(2) Lassaigne’s test. It is a very reliable test for the detection of halogens in an organic compound.
Preparation of the Lassaigne’s extract
(1) Test for halogens. A part of the Lassaigne’s extract is boiled with dil. HNO3 and cooled. A few drops of silver nitrate solution are then added.
(a) A white precipitate soluble in ammonia and insoluble in dil. HNO, indicates the presence of chlorine.
NaCl +AgNO3 ———> AgCl+ NaNO3
(b) A pale yellow precipitate partially soluble in ammonia indicates the presence of bromine.
NaNO3 + AgBr ———> AgBr + NaNO3
c) A yellow precipitate insoluble in ammonia indicates the presence of iodine.
NaI + AgNO3 ——–> AgI + NaNO3
Function of Nitric Acid
If the organic compound also contains nitrogen or sulphur, the Lassaigne’s extract on boiling with dil. HNO3 decomposes sodium cyanide or sodium sulphide formed during fusion.
If cyanide and sulphide ions are not decomposed, they will react with silver nitrate and hence will interfere with the test.
NaCN+AgNO3 ——-> AgCN+ NaNO3
Na2S + 2 AgNO3 ——> Ag2S + 2 NaNO3
(3) Carbon disulphide test for Bromine and Iodine
A small portion of the Lassaigne’s extract is boiled with dil H2SO4 , to decompose sodium cyanide and sodium sulphide. The solution is then cooled and a few mL of freshly prepared chlorine water and carbon disulphide or carbon tetrachloride are added. The solution is vigorously shaken and allowed to stand when:
(1) An orange colour in CS2 or CCl4 layer indicates the presence of bromine.
(2) A violet colour in CS2 or CCl4, layer indicates the presence of iodine.
Theory of the test. Chlorine displaces bromine and iodine from their corresponding halides. The halogen thus liberated dissolves in CS2 or CCl4 , to produce the specific colour.
2 NaBr +Cl2——–>2NaCl +Br2 (Dissolves in CS2 or CCl4 to give orange colour)
2NaI + Cl2——-> 2NaCl+ I2 (Dissolves in CS2, or CCl4, to give violet colour)
Detection of Sulphur
If sulphur is present in the organic compound then on fusion with sodium metal , sodium sulphide is formed.
A small portion of the Lassaigne’s filtrate is treated with a few drops of sodium nitroprusside solution when a violet colouration is obtained. This colour slowly fades on standing.
(b) Lead acetate test.
Another portion of Lassaigne’s filtrate is acidified with dilute acetic acid and a few drops or lead acetate solution are added to it. Formation of black lead acetate the presence of sulphur in the given compound.
Na2S + (CH3COOH)2Pb ———> PbS + 2CH3COONa
(c) Oxidation test
hydrochloric acid and then treated with barium chloride solution when a white precipitate insoluble in hydrochloric acid is obtained.
Detection of Phosphorus
Detection of Oxygen
The compound is tested for the presence of oxygen containing functional group such as -OH, -CHO, -COOH, -NO2 etc. The presence of any one of these groups in the compound, in turn, confirms the presence of oxygen in it.
Very nice elaboration!!
Thank You. It is very informative.
This site just helped me with my homework
Keep it up with the good works