Fission of a covalent bond
Homolytic fission
If a covalent bonds breaks in such a way that each atom takes away one electron of the shared pair, it is called homolytic or symmetrical fission.
Homolytic fission is usually indicated by a fish arrow which denotes a one electron displacement.
For Ex:
The neutral chemical species which contain an odd or unpaired electron and which are produced by homolytic fission of covalent bonds are called free radicals.
Homolytic fission usually occurs in non-polar bonds and is favoured by high temperature , ultraviolet radiations and by the presence of radical indicators such as peroxides.
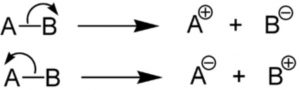
Heterolytic fission
When a covalent bond joining two atoms A and B breaks in such a way that both the electrons of the covalent bond are taken away by one of the bonded atoms, the mode of bond cleavage is called heterolytic fission.
Heterolytic fission is usually indicated by a curved arrow which denotes a two electron displacement.
Heterolytic fission results in the formation of charged species i.e. cation and anion. It usually occurs in polar covalent bond and is favoured by polar solvents.
Inductive effect
Whenever an electron withdrawing atoms such as halogen is attached to the end of a carbon chain, the σ electrons of the C—X bond are attracted by or displaced towards the more electronegative halogen atom. As a result ,the atom X acquires a small negative charge and C1 acquires a small positive charge.
This type of displacement of σ-electron along a saturated carbon chain whenever an electron withdrawing group is present at the end of the chain is called the inductive effect or the -I effect. This effect weakens steadily with increasing distance from the substituent and actually becomes negligible after 3 carbon atoms.
There are two types of inductive effect:
- If the substituent attached to the end of the carbon chain is electron withdrawing the effect is called -I effect.
The -I effect of some of the atoms and groups in the decreasing order w.r.t. to hydrogen is :
NO2 > CN> COOH > F > Cl> Br > I > H
If the subsequent attached to the end of the carbon chain is electron donating the effect is called +I-effect.
of some of the atoms or group in the decreasing order with respect to hydrogen is
Inductive effect is a permanent effect operating in the ground state of the organic molecules and health is responsible for high melting point white and dipole moment of Polar compounds
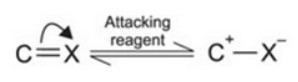
Electromeric effect
It involves the complete transfer of electrons of a multiple bond to one of the bonded atom in presence of an electron attacking reagent. It is called the E effect.
This effect is temporary and takes place only in the presence of a reagent. As soon as the reagent is removed, the molecule reverts back to its original position.
Electromeric effect is of two types:
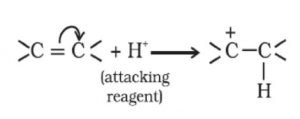
+E effect: If the electrons of the π-bond are transferred to that atom of the double bond to which the reagent gets finally attached, the effect is called +E effect.
For Ex: Addition of acids to alkenes
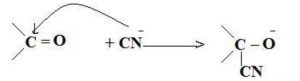
-E Effect : If the electrons of the double bond are transferred to an atom of the double bonds other than the one to which the reagent gets finally attached the effect is called -E Effect.
For Ex: Addition of Cyanide ion to the carbonyl group.
The unsaturated system shows +E effect with electrophiles and -E effect with nucleophiles.

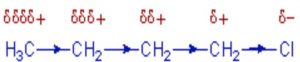
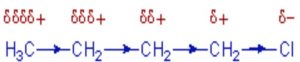
Good job
It was helpful to children
Really, it was very helpful to me because tomorrow , मेरा exam है