Buffer Solution
A buffer solution is defined as a solution which resist any change in its pH value even when small amount of acid or base are added to it.
Types of the buffer solution
1) Solution of single substance
The solution of the salt of weak acid and weak base eg : ammonium acetate ( CH3COOH) act as a buffer.
2) Solution of Mixture:
These are of 2 types:
Acidic buffer : It is the solution of mixture of the weak acid and a salt of this weak acid with a strong base.
For Ex: CH3COOH + CH3COONa or HCOOH + HCOONa
Basic buffer : It is the solution of a mixture of weak base and a salt of this weak base with a strong acid.
For Ex: NH4OH + NH4Cl or NH4OH + NH4NO3
Buffer Action
The property of a buffer solution to resist any change in its pH value even when small amount of the acid or the base are added to it is called Buffer action.
Buffer action of ammonium acetate solution
Ammonium acetate is almost completely dissociated in the aqueous solutions as follow:
CH3COONH4 ————> CH3COO‾ + NH4+
In the solution , there is excess of CH3COO‾ ions and NH4+ ions.
When a few drops of an acid are added to the above solution, the H3O+ ions given by the acid combine with the CH3COO‾ ions to form weakly ionized molecule of CH3COOH.
CH3COO‾ + H3O+—————>CH3COOH + H2O
The H3O+ ion concentration of the solution does not change practically and hence the pH of the solution remains almost constant.
When a few drops of base are added to the above solution the OH‾ ions given by the base combine with NH4+ ions to form some weakly ionized molecules of NH4OH.
NH4+ + OH‾ ———-> NH4OH
The OH‾ ion concentration and hence the H3O+ concentration or the pH of the solution remains almost constant.
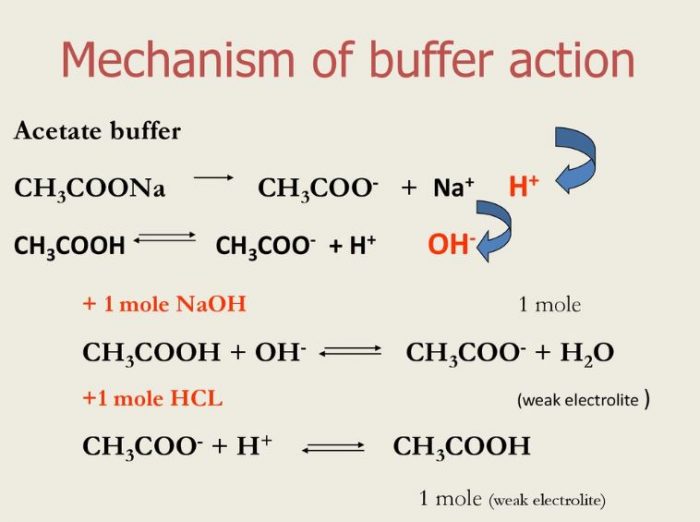
Buffer Action of acidic buffer
Let us consider the buffer action of acidic buffer containing CH3COOH and CH3COONa. Acetic acid dissociates to a small extent whereas sodium acetate is almost completely dissociated in aqueous solution as:
CH3COOH + H2O CH3COO‾ + H3O+ —————-1
CH3COONa ———-> CH3COO‾ + Na+
By common ion effect, the ionization of CH3COOH is further suppressed. Thus in the solution, there are excess of acetate ions and a small amount of H3O+ ions.
When a few drops of an acid are added to the above mixture solution ,the H3O+ ions given by the acid combine with CH3COO‾ ions to form weakly ionized molecules of CH3COOH.
CH3COO‾ + H3O+ ———–> CH3COOH + H2O
The H3O+ ion concentration and hence the pH of the solution remains almost constant.
When a few drops of a base are added, the OH‾ ions given by the base combine with the H3O+ ions already present to form weakly ionized molecules of H2O.
H3O+ + OH‾ ———–>2H2O
As H3O+ ions are consumed ,the equilibrium 1 shifts towards right. Thus more of CH3COOH dissociates to make up the loss of H3O+ ion. Hence the H3O+ ion concentration or pH of the solution does not change.
Buffer action of basic buffer
The buffer action of a basic buffer i.e. NH4OH + NH4Cl may be explained as:
NH4OH dissociates to a small extent whereas NH4Cl dossociates completely in aqueous solution as:
NH4OH NH4+ + OH‾ —————— 1
NH4Cl ————-> NH4+ + Cl‾
By common ion effect the ionization of NH4OH is further suppressed. Thus, in the solution ,there are excess of NH4+ ions and a small amount of OH‾ ions.
When a few drop of a base are added the OH‾ ions given by it immediately combine with NH4+ ions to for weakly ionized NH4OH.
NH4 + + OH‾ ———> NH4OH
The H3O+ ion concentration or pH of the solution remain unaffected.
When a small amount of acid is added ,the H3O+ ions given by its combine with OH‾ ions already produced by NH4OH in equilibrium 1.
As the OH‾ ions are consumed, the equilibrium 1 shifts in forward direction. More of NH4OH dissociates to produce more of OH‾ ions which makes up the loss of OH‾ ion and concentration therefore, the H3O+ ion concentration or pH of the solution remains fairly constant.
It is well to understand the equilibrium chapter. It gives the easy way to very well study. I’m well satisfied in this concept. Thank you very much for the easy concept of this chapter
Thanks! Bidyut for the appreciation.
Thanks it helped me a lot
Thank you very much.. it helped me to understand the concept 🙂
Thanks a lot. These notes are really helpful mam,
Thank for sharing detailed information like this.
Great job..Mrs Shilpi Nagpal thanks alot for wonderful explanation.