Contents
Preparation of Dihydrogen
(1) From water
Dihydrogen may be obtained from water by reduction either with active metals or by electricity.
(a) By the action of water on Active Metals
(1) Cold Water : Very active metals i.e. alkali and certain alkaline earth metals like Na, K , Ca react with water at room temperature evolving dihydrogen.
2Na + 2H2O ———–> 2NaOH + H2
2K + 2H2O ———–> 2KOH + H2
Ca + 2H2O ———–> 2Ca(OH)2 + H2
The reaction with alkali metal is so vigorous and exothermic that the hydrogen involved catches fire. To slow down the reaction, amalgams of these metals are generally used.
In amalgams, only a small surface area of the metal comes in contact with water ,and ,therefore the reaction is slowed down.
(2) Boiling water : Less active metals like Zn, Mg , Al decompose boiling water liberating dihydrogen.
Zn + H2O ———–> ZnO + H2
Mg + H2O ———–> MgO + H2
2Al + 3H2O ———–> Al2O3+ H2
(3) Steam : Still less active metals like Fe, Sn, Ni decompose steam at high temperature evolving dihydrogen.
3Fe + 4H2O ———–> Fe3O4 + H2
steam magnetic oxide
Temperature: 1023-1073 K
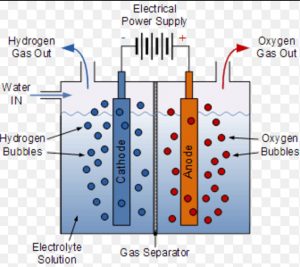
(b) By Electrolysis of Water
Dihydrogen of high purity is usually obtained by the electrolysis of water in the presence of small amount of an acid or a base. During electrolysis, dihydrogen is collected at cathode while dioxygen is liberated at anode.
2H2O (l) ———> 2H2 (g) + O2 (g)
Pure water is only weakly ionized and hence is a poor conductor of electricity but presence of an acid or a base makes it a better conductor of electricity.
This method is not commercially used since it is quite expensive.
(2) From Alkalis
Metals like Be, Zn, Sn react with boiling alkali solution liberating dihydrogen.
Be + 2NaOH ———–> Na2BeO2 + H2
Zn + 2NaOH ———–> Na2ZnO2 + H2
Sn + 2NaOH + H2O———–> Na2SnO3 + H2
2Al + 2NaOH + 2H2O ———-> 2NaAlO2 + 3H2
(3) From acids
Metals which are more electropositive than hydrogen (lie above hydrogen in the electrochemical series) such as Zn, Fe, Mg react with dilute mineral acids to liberate dihydrogen gas.
Zn + H2SO4 ———-> ZnSO4 + H2
Fe +2HCl ———–> FeCl2 + H2
Metals like Copper ,silver ,Mercury which are less electropositive than hydrogen (lie below hydrogen in the electrochemical series) do not liberate hydrogen from acids.
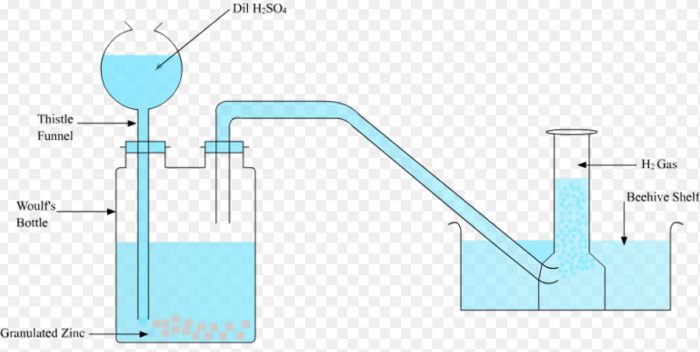
Laboratory preparation of dihydrogen
In laboratory ,dihydrogen is prepared by reaction of dil H2SO4 on granulated zinc.
Zn + H2SO4 ——–> ZnSO4 + H2
Granulated pieces of zinc are placed in a Woulfe’s bottle and are covered with water. The bottle is fitted with a thistle funnel and a delivery tube.
Conc H2SO4 is poured slowly through the thistle funnel. As the acid falls in the Woulfe’s bottle ,it gets diluted and then reacts with zinc evolving dihydrogen gas. It is collected by downward displacement of water.
Sometimes the bubbles of dihydrogen produced stick to the surface of the zinc metal preventing the further reaction of the acid on the metal. Such a situation can be avoided by adding few crystal of copper sulphate to the reaction mixture.
Preparation of Pure Dihydrogen Gas
(1) By the action of pure sulphuric acid on magnesium ribbon
Mg + H2SO4 (dil) ——-> MgSO4 + H2
(2) By the electrolysis of a warm solution of barium hydroxide using platinum or Nickel electrodes.
(3) By the action of water on sodium hydride
NaH + H2O ——–> NaOH + H2
(4) By the action of KOH on scrap aluminium
2Al + 2KOH + 2H2O———-> 2KAlO2 + 3H2
Commercial preparation of Dihydrogen
(1) By the electrolysis of water
A small quantity of acid or alkali is added to water to make it a good conductor and electrolysed in a cell. In this cell ,iron sheet is used as a cathode while nickel plated iron sheet act as anode.
The two electrodes are separated from each other by an asbestos diaphragm which prevents mixing of dihydrogen and dioxygen. On passing electric current, dihydrogen gas is collected at cathode while dioxygen at anode.
When 20% NaOH solution is used for electrolysis, the decomposition of water takes place as follows:
At cathode
H+ + e‾ ———–> H•
H• + H• ———> H2
At anode
4OH‾ ———> 4OH + e‾
4 OH‾ ——–> 2H2O + O2
(2) From Syngas – Bosch process
When super heated steam is passed over red hot coke or coal at 1270K in the presence of nickel catalyst, a mixture of carbon monoxide and dihydrogen is produced.
C(s) + H2O (g) ———-> CO + H2 -121.3 KJ
A mixture 1:1 of CO and H2 was called water gas. All mixtures of CO and H2 irrespective of their composition are called synthetic gas or syngas.
This process of producing syngas from coke or coal is coal gasification.
Syngas is produced from sewage ,sawdust, scrap wood ,newspaper.
It is difficult to obtain pure hydrogen from water gas or Syngas, since CO is difficult to remove. To remove CO and increase the production of dihydrogen from syngas, CO of the syngas is oxidised to CO2 by mixing it with more steam at 673 K in presence of iron chromate as catalyst.
CO ( g) + H2O ( g ) + H2O (g) ————-> CO2 (g) + 2H2(g) 673K, FeCrO4
syn gas . steam
Chemical reaction in which carbon monoxide of the syngas reacts with steam to form carbon dioxide and more dihydrogen is called water gas shift reaction.
CO2 thus produced is removed either by scrubbing the mixture with sodium arsenide solution or bypassing the mixture through water under 30 atm pressure when carbon dioxide dissolves leaving behind dihydrogen which is collected.
(3) From Hydrocarbons
(1) Partial oxidation of hydrocarbons : A mixture of hydrocarbons is mixed with steam and passed over heated Nickel catalyst at 1270 K.
CnH2n+2 + nH2O ————-> nCO + (2n+1) H2
Natural gas may also be used.
CH4 (g) + H2 (g) ——-> CO(g) + 3H2(g)
Whole process of obtaining dihydrogen from natural gas is called steam reforming process.
(2) Thermal cracking of natural gas
Dihydrogen may also be obtained by thermal cracking of natural gas at 1270 K in the presence of a catalyst.
CH4 ——> C + 2H2
(4) Lane’s Process
Dihyrogen can also be manufactured by passing alternate currents of steam and water gas over red hot iron. It consists of two stages:
(1) Oxidation Stage: Super heated steam is passed over iron filling heated to about 1023 – 1073 K when hydrogen is formed and magnetic oxide of iron is left behind.
3Fe + 4H2O ————> Fe3O4 + 4H2 + 160.7 KJ
(2) Reduction stage: When the whole of iron has being oxidised, the steam supply is cut off and a steam of water gas is passed to reduce Fe3O4 back to iron.
Fe3O4+ 4 H2 ———–> 3Fe + 4H2O
Fe3O4+ 4CO———> 3Fe + 4CO2
By passing steam and water gas alternatively over heated iron, dihydrogen gas can be manufactured from a small quantity of iron.
(5) As a by – product
Large quantities of dihydrogen are obtained as a by product in various industries.
(1) From petroleum cracking plants
(2) In the manufacture of sodium hydroxide and chlorine by electrolysis of brine solution.
Anode 2Cl‾ (aq) ————–> Cl2 (g) + 2 e‾
Cathode 2H2O (l) + 2e‾ ——–> H2(g) + OH‾ (aq)
Overall reaction: 2Cl‾ (aq) + 2H2O (l) ———> Cl2 (g) + H2(g) + OH‾ (aq)
Properties of Dihydrogen
(1) It is colourless, tasteless and odourless gas.
(2)It is the lightest substance known.
(3)It is slightly soluble in water since its molecules are non polar.
(4)It can be liquefied under low temperature and high pressure.
Chemical Properties of Dihydrogen
(1) The H-H bond dissociation enthalpy is the highest for a single bond between two atoms of any element.
H2 ( g) ———–> 2H(g)
Dihydrogen is quite stable and relatively inert at room temperature due to its high H-H .bond dissociation enthalpy. Therefore most of the reactions of dihydrogen occur at high temperature.
(2) It is neutral to Litmus.
(3) Combustibility : It is highly combustible gas and burns in air or dioxygen with a pale blue flame to form water. It is not a supporter of combustion.
2H2 ( g ) + O2 (g) ———> 2H2O(l)
(4) Reaction with metals
Dihydrogen reacts with strongly electropositive metals like Sodium ,Potassium ,calcium at high temperature to form salt like metal hydrides, in which the oxidation state of hydrogen is -1.
2Na + H2 ————-> 2NaH
Ca + H2 ————-> CaH2
(5) Reaction with non metals
Dihydrogen combines with many non metals at high temperature in presence of a catalyst to form covalent or molecular hydrides.
(a)With dioxygen ,dihydrogen forms water.The reaction is highly exothermic.
2H2 (g)+ O2 (g) ——–> 2H2O (l) ΔH = – 285.9 Kj mol-1
(b) Reaction with halogens
Dihydrogen combines with halogens to form hydrogen halides.
H2 (g) + X2 (g) ———–> 2HX (g) where X= F, Cl, Br, I
The reactivity of halogens towards dihydrogen decreases in the order:
F2 > Cl2 > Br2 > I2
Fluorine reacts in dark, chlorine in the presence of diffused sunlight, while the reaction with bromine and Iodine occurs on heating in the presence of a catalyst.
H2 (g) + F2 ( g ) ———–> 2HF (g) . dark
H2 (g) + Cl2 ( g ) ———–> 2HCl (g) . 673 K , sunlight
H2 (g) + Br2 ( g ) ———–> 2HBr (g) 673 K , catalyst
H2 (g) + I2 ( g ) ———–> 2HI (g) 673 K , Pt catalyst
(c) With dinitrogen it forms ammonia
N2 (g) + 3 H2 (g) ———-> 2NH3 ( g) 673 K , 200 atm, Fe, Mo , ΔH = -92.6 KJ/ mol
Fe act as a catalyst while Mo act as a promoter. This reaction is used for the manufacture of ammonia by the Haber’s process.
(d) With Sulphur ,it forms hydrogen sulphide.
H2 (g) + S (l) ———-> H2S (g) . 700K
e) With carbon
Depending upon condition it forms Methane or acetylene.
C(s) + 2H2 (g) ———-> CH4 (g) 1375 K
2C (s) + H2 (g) ———–> C≡C electric arc, 3300 K
(6) Reduction of metal oxides and ions
Dihydrogen acts as a reducing agent and hence reduces oxides of certain metals less electropositive than zinc such as those of Cu, Pb, Fe etc to corresponding metals.
CuO + H2 ——-> Cu + H2O
ZnO + H2 ————–> Zn + H2O
PbO + H2 ———> Pb + H2O
Dihydrogen also reduces some metal ions in aqueous solution.
Pd2+ (aq) + H2 (g) ———> Pd (s) + 2 H+ (aq)
Cu 2+ (aq) + H2 (g)———> Cu (s) + 2H+ (aq)
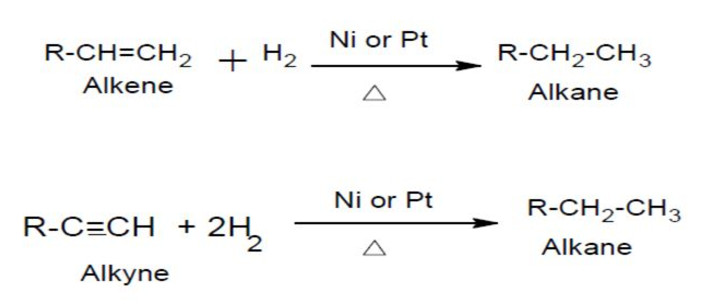
(7) Hydrogenation of unsaturated hydrocarbons
Unsaturated hydrocarbons such as alkenes and alkynes add dihydrogen in presence of a catalyst to form saturated hydrocarbon.
Hydrogenation of unsaturated organic compounds in presence of heterogeneous and homgeneous catalyst is used in many industrial processes.
(8) Hydroformylation of Olefins
Olefins reacts with carbon monoxide and dihydrogen in presence of octacarbonyl dicobalt as catalyst under high temperature and pressure to form aldehydes.
This reaction is called hydroformylation or the oxo- process. The aldehydes thus obtained on subsequent catalytic reduction gives alcohols.
RCH2CH2CHO + H2 ———-> RCH2CH2CH2OH Ni , heat
(9) Hydrogenation of Oils
The vegeatable oil such as soyabean oil, cotton seed oil ,groundnut oil are called polyunsaturated oils since they contain many carbon carbon double bonds.When these oils are exposed to air for prolonged period ,the double bond present in them undergo oxidation and the oils become rancid i.e. develop unpleasant odour and taste due to formation of lower aldehydes and carboxylic acid.
Dihydrogen is bubbled through edible oils in presence of finely divided nickel at 473 K when the oils are converted into solid fats.
Vegetable oil + H2 ——–> Vegeatble fat 473 K , Ni catalyst
This process is called hydrogenation or hardening of oil and is used in the manufacture of vegetable ghee.
Uses of Dihydrogen
Dihydrogen is used
(1) in the manufacture of ammonia which is used for the production of various fertilizers.
(2) in the hydrogenation of vegetable oils to form solid fats
(3) in manufacture of bulk chemical such as methanol
CO(g) + 2H2 (g) ——-> CH3OH (l)
(4) in the manufacture of hydrogen chloride
H2 (g ) + Cl2 (g) ——–> 2HCl (g) sunlight
(5) in the manufacture of metal hydrides
(6) in metallurgy to reduce heavy metal oxides to metal
(7) in the atomic hydrogen and oxy-hydrogen torches for cutting and welding.
(8) Liquid hydrogen is used as a rocket fuel in space research.
(9) It is also used in fuel cells for generating electrical energy.It does not cause any pollution and produces greater energy per unit mass of fuel in comparison to gasoline and other fuels.
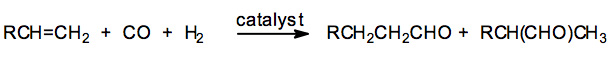
Thank you for the information , it helped me a lot.
Very nice notes
Highly useful
It helped a lot for my exam preparation
Very nice
Extremely great and good and very useful for Class 11 on Preparation Of Hydrogen
Really helpful…..it helped a lot for preparing my exam…goood
Thanks for Sharing knowledge
Very nice notes and very useful.
Thank you so much…
.
Thank you very much, it’s so wonderful!
Thank you mam, God bless you
Very nice